
 English
English
 French
French
Study of the cytokine concentrations in unselected peripheral blood in children with asthma
Étude sur les concentrations des cytokines dans le sang périphérique non sélectionné chez les enfants asthmatiques
H. Le-Thi-Thu1, T. Nguyen-Thi-Dieu2
1: National Vietnam Children’s Hospital. Hanoi, Viet Nam
2: Hanoi Medical University. Hanoi, Viet Nam
Corresponding author
Dr. Huong LE-THI-THU
National Vietnam Children’s Hospital. Hanoi, Viet Nam
E-mail: lehuong199@yahoo.com
DOI: 10.12699/jfvp.7.22.2016.55
ABSTRACT
Background. Asthma is a respiratory disease characterized by chronic airway inflammation involving many cells and cellular components. Cytokines are proinflammatory protein regulated mainly from Th2 cells which play a critical role in activation of airway inflammation. Measurements of cytokine concentration in unselected peripheral blood can predict the inflammatory prognosis in children with asthma exacerbations.
Methods. This was a prospective descriptive study in 55 children with acute asthma, 20 children with stable asthma and 15 healthy children. All subjects were measured IL-4, IL-5 and IL-13 in unselected peripheral blood by flowcytometry-assisted immunoassay methods.
Results. The concentrations of IL-4 and IL-5 were significantly higher in the exacerbation periods in comparison with the stable periods (0.33pg/ml versus 0.036 pg/ml, p=0.08; 2.3 pg/ml versus 1.0 pg/ml, p=0.02; respectively) and healthy controls (0.33pg/ml versus 0.02 pg/ml, p=0.002; 2.3 pg/ml versus 0.75 pg/ml, p=0.029; respectively). There were no difference in the concentrations of IL-4, IL-5 between patients with stable asthma and healthy controls. There were also no difference in concentration of IL-13 in the three groups.
Conclusions. The cytokines such as IL-4, IL-5 play an important role in the activation and maintaining airway inflammation in children with asthma.
KEY-WORDS: Asthma; acute asthma exacerbation; cytokines; IL-4; IL-5; IL-13.
RÉSUMÉ
Contexte. L'asthme est une maladie respiratoire caractérisée par une inflammation chronique des voies aériennes impliquant de nombreuses cellules et des composants cellulaires. Les cytokines sont des protéines pro-inflammatoires régulées principalement à partir de cellules Th2 qui jouent un rôle critique dans l'activation de l'inflammation des voies aériennes . Les mesures de la concentration de cytokines dans le sang périphérique non sélectionné peuvent prédire le pronostic inflammatoire chez les enfants présentant des exacerbations de l'asthme.
Méthodes. Il s'agit d'une étude descriptive prospective chez 55 enfants atteints d' exacerbation aigue de l’asthme, 20 enfants atteints d'asthme stable et 15 enfants sains. Tous les sujets ont été mesurés IL-4, IL-5 et IL-13 dans le sang périphérique non sélectionné par cytofluorométrie assistée immunoessais.
Résultats. Les concentrations d'IL-4 et d'IL-5 étaient significativement plus élevées dans les périodes d'exacerbation que dans les périodes stables (0,33 pg / ml contre 0,036 pg / ml, p = 0,08, 2,3 pg / ml contre 1,0 pg / ml, p = 0,02, respectivement) et des témoins sains (0,33 pg / ml contre 0,02 pg / ml, p = 0,002, 2,3 pg / ml contre 0,75 pg / ml, p = 0,029, respectivement). Il n'y avait aucune différence dans les concentrations d'IL-4, IL-5 entre les patients avec l'asthme stable et les témoins sains. Il n'y avait pas non plus de différence dans la concentration d'IL-13 dans les trois groupes.
Conclusion. Les cytokines telles que IL - 4, IL - 5 jouent un rôle important dans l 'activation et le maintien de l' inflammation des voies aériennes chez les enfants asthmatiques.
MOTS CLÉS: Asthme; cytokines, IL-4; IL-5; IL-13.
INTRODUCTION
Cytokines play a key role in orchestrating the chronic inflammation and structural changes of the respiratory tract in asthma and have become important targets for the development of new therapeutic strategies in these disease. Cytokines are classified into lymphokines, proinflammatory cytokines, growth factors, chemokines and antiinflammatory cytokines, although many of these functions may overlap [1].
In patients with asthma, there is an increase in the number of CD4+ Th cells in the airways, which are predominantly of the Th2 subtype. Th2 cells are characterized by secretion of IL-4, IL-5, and IL-13 [2]. These cytokines from Th2 cells play an important role in maintaining the inflammation in the airways, even if patients do not have clinical symptoms [3]. Measurements of cytokine concentrations in peripheral blood in children with acute and stable asthma help to understand the airway inflammation in asthmatic children.
We conducted this study to compare these cytokine concentrations from Th2 cells in peripheral blood between children in the period of the acute asthma and the asymptomatic period.
PATIENTS AND METHODS
This was a prospective descriptive study in 55 children in acute asthma, 20 children with free symptoms and 15 healthy children. Children with asthma were diagnosed according to international recommendation (GINA-2015) [4]. The severity of asthma was assessed according to Pediatrics Asthma Score (PAS) [5].
Blood samples (3ml) were collected from children with asthma for quantitative of IL-4, IL-5 and IL-13 cytokines at the time of admission and discharge. cyanose, et un wheezing.
Healthy children admitted to hospital for health checks were invited to be volunteer to participate in this study. Blood samples of healthy children were collected only one time. All blood samples collected were drawn into tubes containing 10% ethylenediaminetetra-acetic acid and kept refrigerated at -800C.
Cytokines were quantified by flowcytometry-assisted immunoassay technique with Bio-Plex system from Bio-Rad Laboratories production (USA).
This study was approved by the Medical Ethics Committee of National Vietnam Children’s Hospital. The study results were analyzed using SPSS 16.0 software.
RESULTS
The study enrolled 55 children with acute asthma, 20 children with stable asthma and 15 healthy children which were collected blood samples for quantitative of IL-4, IL-5 and IL-13 concentrations.
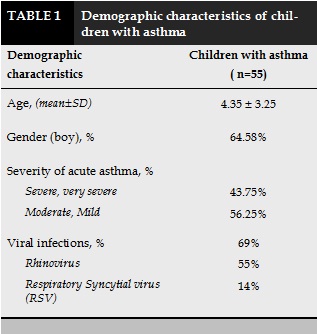
Children hospitalized due to asthma attacks were mainly under 5 years old, with the boys were 64.58%. In acute period, 69% of children with asthma had viral infection, in which 55% having Rhinovirus and 14% having RSV infection. 43.75% of children suffered from severe and very severe asthma exacerbations (Table 1).
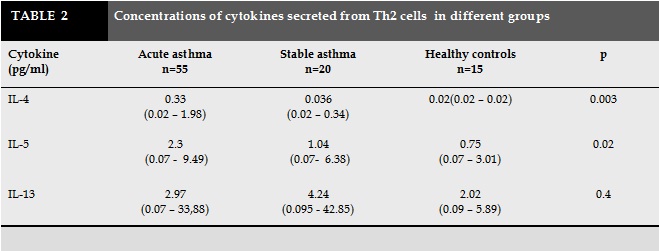
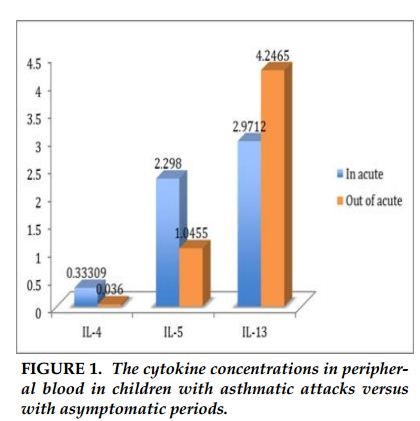
The concentration of IL-4 in children with acute asthma was significantly higher than asthmatic children without symptoms (0.33pg/ml compared to 0.036 pg/ml, p =0.08). Similarly, concentration of IL-5 in acute period was higher than stable period (2.3 pg/ml versus 1.0 pg/ml, p =0.02). There was no difference in IL-13 concentrations between children with acute asthma and asymptomatic period.
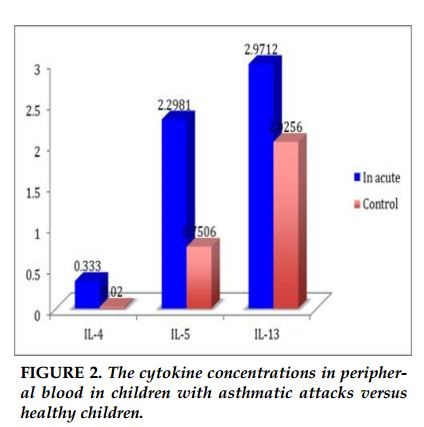
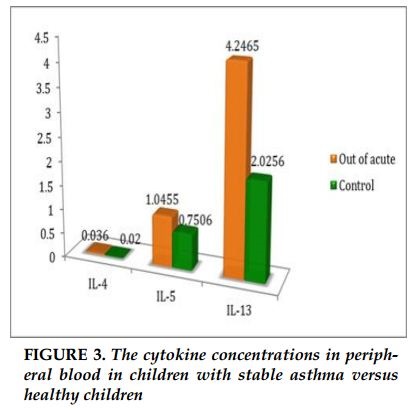
The concentration of IL-4 in acute asthma were significantly higher than the control group (0.33pg/mL versus 0.02 pg/ml, p =0.002). Similarly, concentration of IL-5 in acute asthma was higher than the control group (2.3 pg/mL versus 0.75 pg/ml, p = 0.029). The concentration of IL-13 in children with asthma was no difference in comparison with healthy children (2.9pg/ml versus 2.0 pg/ml). There were no differences in the concentrations of IL-4, IL-5 and IL-13 between children with stable asthma and healthy controls (0.036 pg/mL versus 0.02 pg/ml; p = 0.39; 1.0pg / ml versus 0.75pg / ml; p = 0.79; 4.2pg / ml versus 2.0 pg / ml; p = 0.4; respectively). The concentrations of IL-4, IL-5 in children with acute asthma were significantly increased in comparison with children with stable asthma and healthy controls. The concentration of IL-13 was no different in children with acute asthma, stable asthma and healthy controls.
DISCUSSION
Chronic airway inflammation is the basis pathology of asthma. Studies in animal models and humans indicate the important role of Th2 cells produce IL-4, IL-5, IL-13 [6], the sustained inflammatory cytokines in allergic diseases and allergic asthma. The primary inflammatory lesion of asthma consists of accumulation of CD4 (+) T helper type 2 (Th2) lymphocytes and eosinophils in the airway mucosa.
Th2 cells orchestrate the asthmatic inflammation through the secretion of a series of cytokines, particularly interleukin 4 (IL-4), IL-13, IL-5 [7]. IL-4 is an important cytokine in the progression of allergic inflammation. IL-4 is the major factor regulating IgE production by B cells, and is required for optimal Th2 differentiation [7] [8]. An increase in IL-4 is observed in blood and bronchoalveolar lavage in patients with allergic asthma [9]. IL-4 induces airway hyperresponsiveness in patients with asthma [10].
Interleukin-5 has long been associated with the cause of several allergic diseases including allergic rhinitis and asthma. An increase in IL-5 have been observed in circulating, airway tissue, and induced sputum in patients with asthma. IL-5 is crucial in regulating the eosinophilic response both in vitro and in vivo. IL-5 is a key factor for eosinophilia and could therefore be responsible for some of the tissue damage seen in chronic asthma [7].
Interleukin-13 is a pleiotropic Th2 cytokine that has been shown to be central to the pathogenesis of asthma. Some of the most prominent of the effects of IL-13 include increases in goblet cell differentiation, activation of fibroblasts, elevation of bronchial hyperresponsiveness, and switching of B cell antibody production from IgM to IgE. These data indicate that IL-13 antagonists may fulfill an important unmet need in patients with poorly controlled asthma and biologic evidence of persistent IL-13 activity [11].
Study of Berry showed that the concentration of IL-13 is increased in children with asthma in comparison with healthy children [12]. Our study indicated that the concentrations of IL-14, IL-5 were significantly higher in children with acute asthma in compared to children with stable asthma and healthy controls. However, there were no difference in the concentration of IL-13 in three groups.
The concentration of IL-4 is an increase in blood, serum and bronchoalveolar lavage in patients with asthma [10] [13]. In addition, concentration of IL-4 is often increased in the presence of respiratory syncytial virus [14]. According to Hoekstra, the difference in the concentration of IL-4 seems to depend on the severity of acute asthma [15]. Bogie reported that children having moderate and severe acute asthma had higher IL-4 concentration compared to children with mild acute asthma [13]. However, another study showed no difference in the concentration of IL-4 between the control group and children with mild or moderate acute asthma [16].
According to Charoenying, the concentrations of IL-4 are no difference between patients with acute asthma and patients with stable asthma [17], as well as the control groups [18]. The different results may be explained by the differences in the sample sizes, and the difference of asthma severity in different studies.
The concentration of IL-5 in the serum of patients with asthma is significantly higher than the control groups [13]. IL-5 concentration of children with moderate or severe asthma attacks is significantly higher than the group with mild asthma [9]. However, study by Al Daghri indicated that IL- 5 is reduced in children with severe asthma compared with the control groups, but IL-4 concentration is significantly increased [19].
Although IL-13 has been shown to be central to the pathogenesis of asthma, Machura indicated that IL-13 concentration does not differ between children with asthma and control groups [18]. Al Daghri showed a similar results [19]. Our study did not find the difference in IL-13 concentrations in children with acute asthma, children with stable asthma and healthy children. However, Machura also indicated that IL-13 concentration of CD4 Th2 cells is an increase in children with severe acute asthma, but no variation in children with mild or moderate acute asthma and positively correlation with the duration of asthma [18].
CONCLUSION
These cytokines produced from Th2 cells play an important role in maintaining inflammation in children with asthma. The cytokines such as IL-4, IL-5 are increased in children with acute asthma compared to children with stable asthma and healthy children demonstrated an important role of these cytokines in activating and maintaining the acute period of asthma.
Acknowledgments
We thank the doctors, nurses and the staff of the Department of Allegy- Immunology and Rhematology of the National Vietnam Children’s Hospital had helped us to conduct this study. This study was supported by a grant from the National Foundation for Science & Technology Development (NAFOSTED no. 106.99-2012.12 ).
CONFLIT OF INTEREST
Non.
REFERENCES
1. Barnes PJ (2008). The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest 118: 3546-3556.
2. Barnes PJ (2008). Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8:183-192.
3. Chiba Y, Nakazawa S, Todoroki M et al. (2009), Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol 40: 159–67.
4. GINA 2015. Global strategy for asthma management and prevention.
5. Ducharme FM, Chalut D, Plotnick L, et al (2008). The Pediatric Respiratory Assessment Measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. J Pediatr 152: 476–480.
6. Larché M, Robinson DS and Kay AB (2003). The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 111(3) 450–463.
7. Renauld JC (2001). New insights into the role of cytokines in asthma, J Clin Pathol 54, 577–89.
8. Seder RA, Paul WE, Davis MM et al(1992). The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4 T from T cell receptor transgenic mice, J Exp Med; 176:1091–1098.
9. Walker C, Bauer W, Braun RK et al (1994), Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia, Am J Respir Crit Care Med, 150 (4):1038–1048.
10. Shi HZ, Deng JM, Xu H et al (1998) Effect of inhaled interleukin-4 on airway hyperreactivity in asthmatics, Am JRespir Crit Care Med 157:1818–1821.
11. Corren J (2013). Role of interleukine-13 in asthma. Curr Allergy Asthma Rep. 13(5) 415-20.
12. Berry MA, Parker D, Neale N et al. (2004), Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol 114: 1106–9.
13. Bogie M, Savic N, Tomi V et al (2004) Clinical significance of measurement of interleukin 4 and interleukine 5 serum concentrations in bronchial asthma. Jugoslov Med Biohem. 23: 51–54.
14. Johnson TR, Graham BS (1999). Secreted respiratory syncytial virus Glycoprotein Induces Interleukin-5 (IL-5), IL-13, and Eosinophilia by an IL-4-Independent Mechanism. J Virol, 73(10): 8485-8495.
15. Hoekstra MO, Hoekstra Y, De Reus D et al (1997). Interleukin-4, interferon-gamma and interleukin-5 in peripheral blood of children with moderate atopic asthma . Clin Exp Allergy. 27:1254–1260.
16. Abdulamir AS, Hafidh RR, Abubakar F, Abbas KA (2008). Changing survival, memory cell compartment, and T-helper balance of lymphocytes between severe and mild asthma. BMC Immunology, 9 (73) doi: 10.1186/1471-2172-9-73.
17. Charoenying Y, Kamchaisatian W, Atamasirikul K et al (2010). .Cytokine responses during exacerbation compared with stable phase in asthmatic children. Asian Pacific J Allergy Immunol 28: 35-40
18. Machura E, Mazur B, Rusek-Zychma M, Czarneck MB (2010). Cytokine production by peripheral blood CD4+ and CD8+T Cells in atopic childhood asthma. Clin Develol Immunol. 2010. PMC 3004408.
19. Al- Daghri NM, Alokail MS, Abd-Alrahman SH (2014). Th1/Th2 cytokine pattern in Arab children with severe asthma.Int J Clin Exp Med. 7(8) 2286-91.
TABLES AND FIGURES
RFERENCES
1. Barnes PJ (2008). The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest 118: 3546-3556.
2. Barnes PJ (2008). Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8:183-192.
3. Chiba Y, Nakazawa S, Todoroki M et al. (2009), Interleukin-13 augments bronchial smooth muscle contractility with an up-regulation of RhoA protein. Am J Respir Cell Mol Biol 40: 159–67.
4. GINA 2015. Global strategy for asthma management and prevention.
5. Ducharme FM, Chalut D, Plotnick L, et al (2008). The Pediatric Respiratory Assessment Measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. J Pediatr 152: 476–480.
6. Larché M, Robinson DS and Kay AB (2003). The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 111(3) 450–463.
7. Renauld JC (2001). New insights into the role of cytokines in asthma, J Clin Pathol 54, 577–89.
8. Seder RA, Paul WE, Davis MM et al(1992). The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4 T from T cell receptor transgenic mice, J Exp Med; 176:1091–1098.
9. Walker C, Bauer W, Braun RK et al (1994), Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia, Am J Respir Crit Care Med, 150 (4):1038–1048.
10. Shi HZ, Deng JM, Xu H et al (1998) Effect of inhaled interleukin-4 on airway hyperreactivity in asthmatics, Am J Respir Crit Care Med 157:1818–1821.
11. Corren J (2013). Role of interleukine-13 in asthma. Curr Allergy Asthma Rep. 13(5) 415-20.
12. Berry MA, Parker D, Neale N et al. (2004), Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol 114: 1106–9.
13. Bogie M, Savic N, Tomi V et al (2004) Clinical significance of measurement of interleukin 4 and interleukine 5 serum concentrations in bronchial asthma. Jugoslov Med Biohem. 23: 51–54.
14. Johnson TR, Graham BS (1999). Secreted respiratory syncytial virus Glycoprotein Induces Interleukin-5 (IL-5), IL-13, and Eosinophilia by an IL-4-Independent Mechanism. J Virol, 73(10): 8485-8495.
15. Hoekstra MO, Hoekstra Y, De Reus D et al (1997). Interleukin-4, interferon-gamma and interleukin-5 in peripheral blood of children with moderate atopic asthma . Clin Exp Allergy. 27:1254–1260.
16. Abdulamir AS, Hafidh RR, Abubakar F, Abbas KA (2008). Changing survival, memory cell compartment, and T-helper balance of lymphocytes between severe and mild asthma. BMC Immunology, 9 (73) doi: 10.1186/1471-2172-9-73.
17. Charoenying Y, Kamchaisatian W, Atamasirikul K et al (2010). .Cytokine responses during exacerbation compared with stable phase in asthmatic children. Asian Pacific J Allergy Immunol 28: 35-40
18. Machura E, Mazur B, Rusek-Zychma M, Czarneck MB (2010). Cytokine production by peripheral blood CD4+ and CD8+T Cells in atopic childhood asthma. Clin Develol Immunol. 2010. PMC 3004408.
19. Al- Daghri NM, Alokail MS, Abd-Alrahman SH (2014). Th1/Th2 cytokine pattern in Arab children with severe asthma. Int J Clin Exp Med. 7(8) 2286-91.
ARTICLE INFO
DOI: 10.12699/jfvp.7.22.2016.55
Conflict of Interest
Non
Date of manuscript receiving
12/10/2016
Date of publication after correction
25/11/2016
Article citation
Le-Thi-Thu. H, Nguyen-Thi-Dieu. T. Study of the cytokine concentrations in unselected peripheral blood in children with asthma. J Func Vent Pulm 2016;22(7):49-53.