
 English
English
 French
French
Chemotherapy use at the end of life in metastatic non small cell lung cancer: practical analysis
Utilisation de la chimiothérapie en fin de vie dans le cancer du poumon métastatique non à petites cellules: analyse de la pratique
S. Bacha, SE. Oueslati, S. Habibech
Department of Respiratory Medicine 2
Abderruhman Mami University Hospital. Ariana Tunisia
Corresponding author
Dr. Saoussen Bacha
Department of Respiratory Medicine 2. Abderruhman Mami University
Hospital Ariana Tunisia.Street Abbes Ibn Ferness
El Ghezala, Ariana 2083 Tunisia
Email: saoussenbacha@yahoo.fr
DOI: 10.12699/jfvpulm.8.25.2017.8
ABSTRACT
Background. End of life cancer care has become more aggressive. Scarce studies have explored the factors associated with the aggressiveness of cancer care near the end of life (EOL) in non-small cell lung cancer (NSCLC) patients. The aim of the study was to evaluate chemotherapy use at the EOL in patients with primary metastatic NSCLC.
Methods. We conducted a retrospective study which included expired patients diagnosed with primary metastatic NSCLC and who died within 3 months of diagnosis between January 2007 and December 2013 in our thoracic oncology unit.
Results. A total of 183 patients were included. Median age of the patients was 60 years old. The proportion of patients who received chemotherapy in the last three months of life was 31.9%, in the last month 10.4% and in the last two weeks 6.5%. Two point five percent of the patients had a new chemotherapy regimen in the last month of life (LMOL). Patients who didn’t undergo chemotherapy in the LMOL had a median overall survival (OS) of 10.36 months, while median OS for patients who received chemotherapy in the LMOL was 6.86 months (p=0.128). Median survival for patients who didn’t undergo chemotherapy in the last 14 days before death was 9.96 months while median survival for patients who underwent chemotherapy in the last 14 days was 6.83 months (p=0.763).
Conclusion. Our results showed that no survival benefit was found in continuing chemotherapy in the LMOL. Further study is needed to better identify predictors of aggressive care near the EOL.
KEYWORDS: End of life, lung cancer, chemotherapy.
RÉSUMÉ
Contexte. Les soins contre le cancer en fin de vie sont devenus plus agressifs. De rares études ont exploré les facteurs associés à l'agressivité des soins contre le cancer en fin de vie (FDV) chez les patients atteints d'un cancer du poumon non à petites cellules (CPNPC). Le but de l'étude était d'évaluer l'utilisation de la chimiothérapie à la FDV chez les patients atteints de CPCNC métastatique primaire.
Méthodes. Nous avons mené une étude rétrospective incluant des patients périmés diagnostiqués avec un CPNPC métastatique primaire et décédés dans les 3 mois suivant le diagnostic entre janvier 2007 et décembre 2013 dans notre unité d'oncologie thoracique.
Résultats. Un total de 183 patients ont été inclus. L'âge médian des patients était de 60 ans. La proportion de patients ayant reçu une chimiothérapie au cours des trois derniers mois de vie était de 31,9%, le mois dernier de 10,4% et les deux dernières semaines de 6,5%. Deux points cinq pour cent des patients ont eu un nouveau régime de chimiothérapie au cours du dernier mois de vie (DMDV). Les patients qui n'ont pas suivi de chimiothérapie dans le DMDV avaient une survie globale médiane (SG) de 10,36 mois, tandis que la SG médiane pour les patients ayant reçu une chimiothérapie dans le DMDV était de 6,86 mois (p=0,128). La médiane de survie des patients n'ayant pas suivi de chimiothérapie au cours des 14 jours précédant le décès était de 9,96 mois alors que la survie médiane des patients ayant subi une chimiothérapie au cours des 14 derniers jours était de 6,83 mois (p=0,763).
Conclusion. Nos résultats ont montré qu'aucun avantage de survie n'a été trouvé dans la chimiothérapie continue dans le DMDV. D'autres études sont nécessaires pour mieux identifier les prédicteurs de soins agressifs près de la FDV.
MOTS CLÉS: Fin de vie, cancer du poumon, chimiothérapie.
INTRODUCTION
Metastatic NSCLC is a debilitating disease that results in higher burden of symptoms and poor quality of life (QoL). Palliative chemotherapy (PCT) at this stage provides good control of symptoms improves quality of life and prolongs survival [1-3]. Nevertheless, for metastatic NSCLC with a median survival of few months, the end of life (EOL) period was defined as the last month of life (LMOL) [4]. Toxic adverse effects at this breakpoint may overcome the expected benefits of unfortunately useless chemotherapy leading to a reduced patient’s QoL, which is inacceptable when the aim of treatment is palliation of symptoms [5]. However, nowadays, with the spread of new and less toxic molecules, many patients receive chemotherapy too near death, in spite of the fact that lung cancer is resistant to chemotherapy at the EOL [6,7]. The majority of studies working on this issue identified protracted use of chemotherapy as an indicator of aggressive and poor quality of care at the EOL [1,8-10]. Different teams from different countries investigated the quality of EOL care as well as factors and outcomes of aggressive care near the EOL. However these studies focus on various types of solid tumors those dedicated to NSCLC are very few. Hence, we conducted this retrospective study to analyze the use of chemotherapy at the EOL in patients who died of metastatic NSCLC.
MATERIALS AND METHODS
Patient selection
This study was conducted as a single center retrospective cohort study at the thoracic oncology unit of our department. All patients who died of metastatic NSCLC and treated with palliative intent between January 2007 and December 2013 were included. Records of all patients who lived beyond 3 months since the diagnosis and expired were included. Data gathered from patient medical records included the following information: Patient sociodemographic characteristics, palliative chemotherapy patterns and date of death. The indicators of EOL chemotherapy used in this study included: proportion of patients receiving chemotherapy within 30 days of death; proportion of patients starting a new chemotherapy regimen within 30 days of death; proportion of patients receiving chemotherapy within 14 days of death; proportion of patients starting a new chemotherapy regimen within 14 days of death.
RESULTS
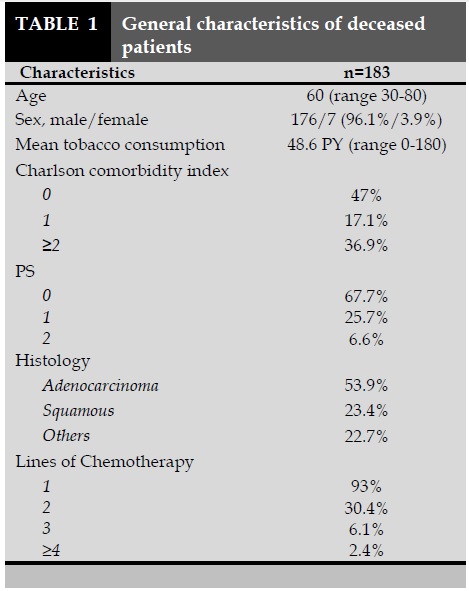
One hundred and eighty three patients with NSCLC died from 2007 to 2013 in our clinic. The median age of the patients was 60 years old. The vast majority of the patients (67.7%) had a PS=0 at the first examination. Table 1 summarizes the characteristics of the patients. Death occurred within the last 3 months after the last chemotherapy cycle in 57 patients (31.9%). Nineteen patients (10.4%) died within one month following the last chemotherapy cycle. Twelve patients (6.5%) died within the 14 days after the last chemotherapy cycle. Two of them (1.1%) died the same day of the last chemotherapy cycle. Median time between last chemotherapy cycle and death was 85.5 days. Median time between last chemotherapy cycle and death in the LMOL chemotherapy group was 12 days. Among the patients who received chemotherapy in the LMOL (n=19), 10 patients (52.6%) were receiving their first line and 7 patients (36.8%) were receiving their second line. Six patients (50%) of those who received their last cycle within the 14 days prior to death were on their first line. Four patients (2.2%) received a new chemotherapy regimen one month before death happened. Two patients (1.1%) were given a new chemotherapy regimen fourteen days before death.
Survival
The median overall survival was 9.9 months. Median survival time for patients who didn’t undergo palliative chemotherapy in the last 3 months of life was 12.16 months while median survival for patients who underwent last 3 months chemotherapy was 6.86 months (p=0.000) (Figure 1).
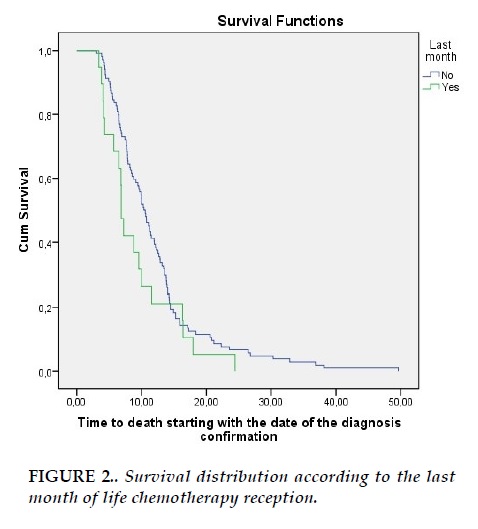
Patients who didn’t undergo chemotherapy in the LMOL had a median overall survival (OS) of 10.36 months, while median OS for patients who received chemotherapy in the LMOL was 6.86 months (p=0.128) (Figure 2).
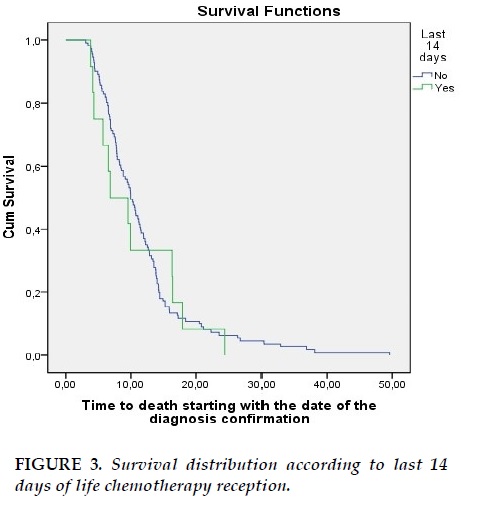
Median survival for patients who didn’t undergo chemotherapy in the last 14 days before death was 9.96 months while median survival for patients who underwent chemotherapy in the last 14 days was 6.83 months (p=0.763) (Figure 3).
DISCUSSION
In this study, we didn’t detect any survival benefit from continuing chemotherapy close to death. Nineteen patients (10.4%) received PCT during the LMOL, among them 12 patients (6.5%) underwent chemotherapy within the last 14 days before death.
The review of the literature showed that rates of chemotherapy administration near the EOL varied widely. The Proportion of patients receiving LMOL chemotherapy ranged from 3.4 to 55.6% in studies including different types of tumors and from 3 to 33.8% for the last 14 days before death [5,9-11,14-27].
The rates of chemotherapy administration within the last 30 and 14 days of life were very high as 55 and 33.8% in Spain [11], 55 and 22% in France [4], 43 and 20% in USA [12]. Earle CC and al determined that health care systems not providing overly aggressive care could be ones in which less than 10% of patients receive chemotherapy in the last 14 days of life, less than 2% start a new chemotherapy regimen in the last 30 days of life [13].
These studies were very heterogeneous, they were conducted in different countries in the world and even administrated drugs were different (classic intravenous chemotherapy, oral treatment, targeted therapy). Besides, authors included various types of cancer. In studies focusing on NSCLC patients, these rates varied from 27.7 % to 55% for the LMOL chemotherapy and from 8.5% to 25% for the last 14 days of life chemotherapy [4,12,28-34].
High rates of chemotherapy utilization at the EOL could be explained by many factors. One explanation for the overuse of chemotherapy at the EOL consists in health system. Warren JL and al, compare care at the EOL for elderly NSCLC patients who died of cancer in the USA and Ontario.
Both groups used health-care services extensively in the last 5 months of life, particularly during the LMOL. Chemotherapy was given to significantly more SEER-Medicare patients than Ontario patients (33% in the USA versus 9% in Ontario) [28].
Furthermore, the availability of new drugs with less toxic side effects and easier administration made the possibility to prescribe drugs very closely to death. Targeted therapy (TT) was frequently initiated within the last 3 months of life and close to death. Wong and al found that among the 36 patients receiving systemic therapy, 12 (33.3%) and 9 patients (25%) received TT respectively in the LMOL and in the last 2 weeks of life. TT continued to be prescribed to patients as close as 5 days before demise. TT in particular EGFR TKIs, are the drug of choice in the systemic treatment of NSCLC at the EOL [33]. Another explanation for over use of chemotherapy at the EOL consists in physicians practice style [35, 36].
Kay S and al found that the individual treating medical oncologist was the only significant predictor for continuing palliative chemotherapy in the LMOL [5]. The doctors are overoptimistic and can’t estimate in an accurate way the right time of dying. Predicting who is going to die of metastatic disease is difficult especially in lung cancer which is known for its fast progression.
Kao S and al found that chemoresponsiveness was a predictor for LMOL chemotherapy [5]. Martoni AA reported that the majority of the patients belonging to the low sensitivity group had been treated in their last 6 months of life [9]. While in several others studies chemotherapy responsiveness did not seem to influence whether dying patients receive chemotherapy at the EOL [13,25,37].
These results suggest that physicians prescribe chemotherapy even when the tumor chemoresponsiveness is low which could be explained by empathy and compassion [23].
Moreover, cancer patients are more likely to receive EOL care that is consistent with their preferences when they have the opportunity to discuss their wishes for EOL care with their physician. Patients who had EOL discussion with their physician before the last 30 days of life were less likely to receive aggressive measures at EOL including chemotherapy [38,39].
However, patients would choose chemotherapy despite the small benefit and the toxicities that could threaten their lives and one of the reasons for starting chemotherapy for very advanced disease is the patient’s request. Best supportive care is often perceived as doing nothing [31].
In our study, median time between last chemotherapy cycle and death was 85.5 days (2.85 months) and it was 12 days in LMOL chemotherapy group. Although other studies included different types of cancer, number of days between last chemotherapy cycle and death for the LMOL chemotherapy group was similar. Sezguin Goksu S and his collaborators found that median time between the last chemotherapy cycle and death in the LMOL chemotherapy group was 15 days [23]. Young J and al found that median time from last administration of systemic anti cancer treatment to death was 16 days in the LMOL group [27]. Sesé L and al found that median time between the last day of active treatment and death was 0.9 months [4].
However, Median time between last chemotherapy cycle and death was 2.02 months in a study conducted in Korea [14], similarly Maartoni AA observed 71 days (2.37 months) between last chemotherapy cycle and death [1]. Gonçalves JF found the longest interval between last chemotherapy cycle and death (168 days) [21]. One possible explanation of the results reported by Gonçalves JF is that the center where his study was conducted included a 20-bed palliative care unit (PCU). Thirty four percent of the patients were admitted to the PCU, where they were monitored from 1 to 1,338 days [21, 40].
Earle CC and al noted that average days between last chemotherapy dose and death decreased from 71 days in 1993 to 65.3 days in 1996 (P<0.001) which illustrates that care at the end of life is becoming more and more aggressive [20]. In our study, we didn’t detect any survival benefit from continuing chemotherapy close to death.
Näppä U and al also found that patients who received palliative chemotherapy during the LMOL had a significantly shorter survival time from starting palliative treatment to death [15]. Saito AM and coworkers reported that the use of chemotherapy within 14 days of death was associated with worse survival at 1 year compared to standard chemotherapy [31].
Patients may also be deprived of good palliative care provided by hospice [31]. Roeland E and collaborators indicated that despite our good intentions, more chemotherapy at the EOL may not prolong life; rather, it may hasten death [3]. Some limitations should be noted in this study. First, it was a retrospective study with a small sample size.
Thus we could not identify factors influencing the prescription of chemotherapy at the EOL. Second, it was a single center study reflecting limited number physician attitudes. Moreover, we could not know the precise interactions that occurred between patients or their family and doctors. Nevertheless, to our knowledge, there is no data in the medical literature concerning the aggressiveness of lung cancer care near the EOL in Tunisia and North Africa.
CONCLUSION
Our study suggests that continuing chemotherapy to near death in metastatic NSCLC do not prolonged survival. Research is warranted to investigate factors influencing the appropriate timing for the discontinuation of palliative chemotherapy in order to reduce costs of futile chemotherapy, minimize the ‘medicalization’ of death and improve QOL at the EOL. Besides, permanent monitoring of quality of care trends is important in order to provide terminally ill cancer patients the best care. In addition, tools to better communicate with patients about prognoses and the balance between benefits and complications of chemotherapy are needed.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Wu CC, Hsu TW, Chang CM, Lee CH, Huang CY, Lee CC. Palliative chemotherapy affects aggressiveness of end-of-life care. Oncologist 2016; 21(6): 771-7.
2. McCall K, Johnston B. Treatment options in end-of-life care: the role of palliative chemotherapy. Int J Palliat Nurs 2007; 13(10): 486-8.
3. Roeland E, Loprinzi C, Moynihan TJ, Smith TJ, Temel J. In chemotherapy for lung cancer, sometimes less is more. J Natl Compr Canc Netw 2013; 11(3): 232-5.
4. Sesé L, Didier M, Rousseau-Bussac G, Crequit P, Masanes MJ, Chouaid C. Chimiothérapie et fin de vie dans les cancers broncho-pulmonaires: analyse des pratiques. Rev Mal Respir 2014; 32(3): 1-6.
5. Kao S, Shafiq J, Vardy, Adams D. Use of chemotherapy at end of life in oncology patients. Ann Oncol 2009; 20(9): 1555-9.
6. Yun YH, Kwak M, Park SM, Kim S, Choi JS, Lim HY et al. Chemotherapy use and associated factors among cancer patients near the end of life. Oncology 2007; 72(3-4): 164-71.
7. Heo DS. Life-sustaining medical treatment for terminal patients in Korea. J Korean Med Sci 2013; 28(1): 1-3.
8. Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 2003; 21(6): 1133-8.
9. Martoni AA, Tanneberger S, Mutri V. Cancer chemotherapy near the end of life: the time has come to set guidelines for its appropriate use. Tumori 2007; 93(5): 417-22.
10. Rautakorpi LK, Seyednasrollah F, Mäkelä JM, Hirvonen OM, Laitinen T, Elo LL and al. End-of-life chemotherapy use at a Finnish university hospital: a retrospective cohort study. Acta Oncol 2017; 31: 1-5.
11. Sanz Ortiz J. Chemotherapy at the end of life: up until when? Clin Transl Oncol 2012; 14(9): 667-74.
12. Murillo JR, Koeller J. Chemotherapy given near the end of life by community oncologists for advanced non-small cell lung cancer. Oncologist 2006; 11(10): 1095-9.
13. Earle CC, Neville BA, Landrum MB, Souza JM, Weeks JC, Block SD et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 2005; 17(6): 505-9.
14. Keam B, Oh DY, Lee SH, Kim DW, Kim MR, Im SA et al. Aggressiveness of cancer-care near the end-of-life in Korea. Jpn J Clin Oncol 2008; 38(5): 381-6.
15. Näppä U, Lindqvist O, Rasmussen BH, Axelsson B. Palliative chemotherapy during the last month of life. Ann Oncol 2011; 22(11): 2375-80.
16. Andreis F, Rizzi A, Rota L, Meriggi F, Mazzocchi M, Zaniboni A. Chemotherapy use at the end of life. A retrospective single centre experience analysis. Tumori 2011; 97(1): 30-4.
17. Braga S, Miranda A, Fonseca R, Passos-Coelho JL, Fernandes A, Costa JD et al. The aggressiveness of cancer care in the last three months of life: a retrospective single centre analysis. Psychooncology 2007; 16(9): 863-8.
18. Hashimoto K, Yonemori K, Katsumata N, Hotchi M, Kouno T, Shimizu C et al. Factors that affect the duration of the interval between the completion of palliative chemotherapy and death. Oncologist 2009; 14(7): 752-9.
FIGURES/TABLES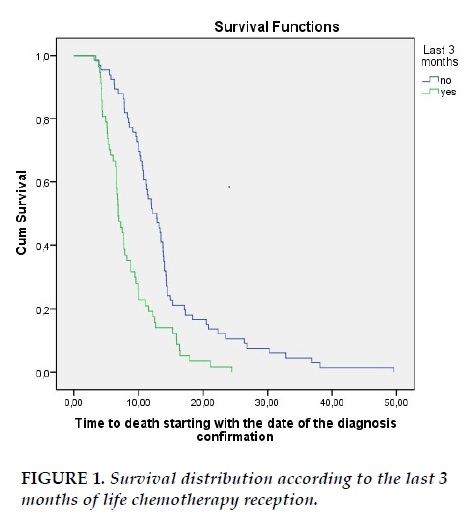
REFERENCES
1. Wu CC, Hsu TW, Chang CM, Lee CH, Huang CY, Lee CC. Palliative chemotherapy affects aggressiveness of end-of-life care. Oncologist 2016; 21(6): 771-7.
2. McCall K, Johnston B. Treatment options in end-of-life care: the role of palliative chemotherapy. Int J Palliat Nurs 2007; 13(10): 486-8.
3. Roeland E, Loprinzi C, Moynihan TJ, Smith TJ, Temel J. In chemotherapy for lung cancer, sometimes less is more. J Natl Compr Canc Netw 2013; 11(3): 232-5.
4. Sesé L, Didier M, Rousseau-Bussac G, Crequit P, Masanes MJ, Chouaid C. Chimiothérapie et fin de vie dans les cancers broncho-pulmonaires: analyse des pratiques. Rev Mal Respir 2014; 32(3): 1-6.
5. Kao S, Shafiq J, Vardy, Adams D. Use of chemotherapy at end of life in oncology patients. Ann Oncol 2009; 20(9): 1555-9.
6. Yun YH, Kwak M, Park SM, Kim S, Choi JS, Lim HY et al. Chemotherapy use and associated factors among cancer patients near the end of life. Oncology 2007; 72(3-4): 164-71.
7. Heo DS. Life-sustaining medical treatment for terminal patients in Korea. J Korean Med Sci 2013; 28(1): 1-3.
8. Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 2003; 21(6): 1133-8.
9. Martoni AA, Tanneberger S, Mutri V. Cancer chemotherapy near the end of life: the time has come to set guidelines for its appropriate use. Tumori 2007; 93(5): 417-22.
10. Rautakorpi LK, Seyednasrollah F, Mäkelä JM, Hirvonen OM, Laitinen T, Elo LL and al. End-of-life chemotherapy use at a Finnish university hospital: a retrospective cohort study. Acta Oncol 2017; 31: 1-5.
11. Sanz Ortiz J. Chemotherapy at the end of life: up until when? Clin Transl Oncol 2012; 14(9): 667-74.
12. Murillo JR, Koeller J. Chemotherapy given near the end of life by community oncologists for advanced non-small cell lung cancer. Oncologist 2006; 11(10): 1095-9.
13. Earle CC, Neville BA, Landrum MB, Souza JM, Weeks JC, Block SD et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 2005; 17(6): 505-9.
14. Keam B, Oh DY, Lee SH, Kim DW, Kim MR, Im SA et al. Aggressiveness of cancer-care near the end-of-life in Korea. Jpn J Clin Oncol 2008; 38(5): 381-6.
15. Näppä U, Lindqvist O, Rasmussen BH, Axelsson B. Palliative chemotherapy during the last month of life. Ann Oncol 2011; 22(11): 2375-80.
16. Andreis F, Rizzi A, Rota L, Meriggi F, Mazzocchi M, Zaniboni A. Chemotherapy use at the end of life. A retrospective single centre experience analysis. Tumori 2011; 97(1): 30-4.
17. Braga S, Miranda A, Fonseca R, Passos-Coelho JL, Fernandes A, Costa JD et al. The aggressiveness of cancer care in the last three months of life: a retrospective single centre analysis. Psychooncology 2007; 16(9): 863-8.
18. Hashimoto K, Yonemori K, Katsumata N, Hotchi M, Kouno T, Shimizu C et al. Factors that affect the duration of the interval between the completion of palliative chemotherapy and death. Oncologist 2009; 14(7): 752-9.
ARTICLE INFO
DOI: 10.12699/jfvpulm.8.25.2017.8
Conflict of Interest
Non
Date of manuscript receiving
15/9/2017
Date of publication after correction
25/12/2017
Article citation
Bacha S, Oueslati SE, Habibech S. Chemotherapy use at the end of life in metastatic non small cell lung cancer: practical analysis. J Func Vent Pulm 2017;25(8):8-13