
 English
English
 French
French
Bronchopulmonary cancer and chronic lymphocytic leukemia: a unique combination for which prognosis?
Le cancer bronchopulmonaire et la leucémie lymphoïde chronique: une combinaison unique pour laquelle le pronostic?
I. Issoufou¹, L. Belliraj¹, F. Ammor1, H. Harmouchi1, M. Lakranbi¹, S. Rachid2,3, Y. Ouadnouni1,4, M. Smahi1,4
1: Thoracic Surgery. Teaching Hospital Hassan II. Fez 30000 Morocco
2: Department of General Surgery, HNN. Niamey, Niger
3: Faculty of Health Science. Abdou Moumouni University. Niamey, Niger
4: University Sidi Mohamed Ben Adellah. Faculty of Medicine and Pharmacy. Fez, Morocco
Corresponding author
Dr. Issoufou IBRAHIM
Thoracic Surgery. Teaching Hospital Hassan II. 1 Rue Nador, Hay Amal Road Sefrou, 30000 Fez Sais Morocco
Email: alzoumib84@gmail.com
DOI: 10.12699/jfvpulm.9.26.2018.40
ABSTRACT
The association of lung cancer and chronic lymphocytic leukemia has already been described by some authors.
The central issue remains the prognosis of both diseases, would it be worse or not by the association, or does there exist a causal link between the two diseases. We report the case of 56 year old, chronic smoker, with a bronchopulmonary cancer and being followed-up for chronic lymphocytic leukemia. He benefited from a right upper lobectomy preceded by a radical mediastinal lymphadenectomy with uncomplicated postoperative outcome. We focus on a systematic search of these secondary cancers to establish early treatment strategies to improve prognosis.
KEYWORDS: Lung Neoplasm, lymphocytic leukemia, prognosis, lobectomy, thoracotomy.
RÉSUMÉ
L'association du cancer du poumon et de la leucémie lymphoïde chronique a déjà été décrite par certains auteurs. La question centrale reste le pronostic des deux maladies, serait-il pire ou non par l'association, ou existe-t-il un lien de causalité entre les deux maladies. Nous rapportons le cas d'un fumeur chronique âgé de 56 ans, atteint d'un cancer bronchopulmonaire et suivi d'une leucémie lymphoïde chronique. Il a bénéficié d'une lobectomie supérieure droite précédée d'une lymphadénectomie médiastinale radicale avec un résultat postopératoire non compliqué. Nous nous concentrons sur une recherche systématique de ces cancers secondaires afin d'établir des stratégies de traitement précoce pour améliorer le pronostic.
MOTS CLÉS: Tumeur pulmonaire, leucémie lymphocytaire, pronostic, lobectomie, thoracotomie.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most common leukemia in Europe representing 1% of cancers while lung cancer would be around 12.9%, responsible for 21% of cancer deaths [1,2]. The association of lung cancer and CLL was described by some authors [3]. The central issue remains the prognosis of both diseases, would it be worse or not by the association or does there exist a cause and effect between the two. Through observation we report on this rare association.
CASE REPORT
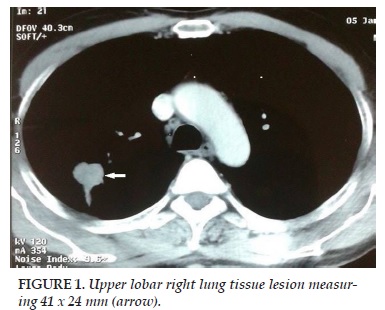
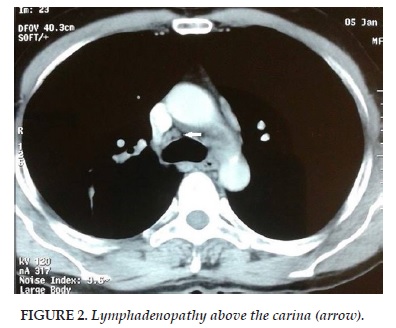
Mr O.A aged 56, chronic smoker for 40 years, presented to our department for stage II Sadoul dyspnea evolving since 3 months. He was being followed for chronic lymphocytic leukemia (CLL) Stage B in remission and had already received six courses of rituximab, fludarabine and cyclophosphamide (RFC). Clinical examination found a patient in good condition with a WHO score 0. A chest radiograph showed a mid-thoracic opacity with central spiculated contours. A CT brain-thorax-abdomen and pelvis was performed. It showed an upper lobar right lung tissue lesion measuring 41 x 24 mm (Figure 1) with ipsilateral hilar lymphadenopathy above the carina (Figure 2) and abdominal. A scan-guided biopsy was performed on the lung tissue lesion. Pathological findings in favor of a primitive lung infiltrating adenocarcinoma. After discussion of the patient file in a multidisciplinary meeting group, surgery was decided. The approach was the right posterolateral conservative thoracotomy. Exploration of an upper right lobe tumor limited to the lobe without invasion of adjacent structures was done. Mediastinal lymph node dissection with frozen section was the first approach. The results excluded pathological tumor in these lymph nodes.
A right upper lobectomy was performed with protection of the bronchial section with a pleural flap. The postoperative course was uneventful and the patient was discharged on postoperative day 4.
DISCUSSION
Chronic lymphocytic leukemia (CLL) is characterized by lymphocytosis a malignant monoclonal proliferation of mature B lymphocytes especially greater than 5x109/L for at least 3 months [1]. Its prevalence increases with age, more than 5% after 60 years. The combination of a concomitant CLL or in following chemotherapy for CLL has been shown by several authors [4]. There is a consensus in the literature about the increased risk of a secondary cancer in patients with chronic lymphocytic leukemia.
In an Australian study, Royle found a doubling of risk of developing a second cancer in patients with CLL. [5] For other authors, younger patients are the most affected with a male predominance [4]. Preferred sites are the colon, rectum, skin (melanoma), prostate, kidneys and lungs [5]. Hisada meanwhile found a prevalence of melanoma and lung cancer [6]. Among these, adenocarcinoma is the most common [4]. They would be responsible for 2% of deaths in patients with CLL [7]. One reason for this increase in secondary cancers is linked to early detection because of prolonged surveillance of the CLL. The second reason is controversial and would be linked to the nature of the treatment of CLL
Thus the role of fludarabine has been implicated by some authors [8] including Benjamini in a study that compiled 234 cases of CLL and showed an increased risk of secondary cancer after chemotherapy with rituximab, fludarabine, and cyclophosphamide [9]. This protocol was the one used in our patient.
A dose-response action was noted. Thus the risk is reduced when the cumulative dose of cyclophosphamide is less than 11250 mg / m 2 [9]. The secondary immunosuppression would be responsible for the increase of secondary cancer because it was also generally observed in renal transplant patients under immunosuppressive therapy. [5] But the biology and genetics common to these cancers could not be excluded.
Indeed, overexpression of HER2 / neu in non-smoking patients with lung cancer and CLL made a reasonable correlation for which Potti et al proposed a systematic search of all lung cancer patients with CLL [10]. And above and beyond the search for this cancer, one of the problems is to be able to classify and stage it according to the TNM classification for determining its status that may be biased because of the possibility of lymph node involvement by CLL. Thus, the discovered nodes, will they be considered as metastatic lung cancer or rather within the scope of the CLL? It is known that PET CT has better performance for judging lymph node tumor involvement. Its role in the CLL was especially clear to discriminate between point A and B Binet classification [11]. But in the case of an association of two potential lymph node malignancies, careful attention deserves to be made as to its interpretation.
Thus the fact that the lymph node lesions of CLL are mostly bilateral and symmetrical, they will be bilateral cervical, bilateral axillary bilateral clavicular addition, bilateral hilar, bilateral inguinal and bilateral external iliac involvement. The first fact could be a discriminative argument between CLL and lung cancer. But latero-tracheal and anterior mediastinal level this rule is not always respected as the lymph nodes may be asymmetrical [11]. Thus the difference could also be on the extent of the Max SUV. Indeed in lung cancer the threshold of the SUVmax remains higher. This value ranges from 5 to 15 in the lung cancer while it is at most 2.74 in the CLL particularly in Stage B [11]. Still, the certainty of a lymph node involvement could only be obtained by histology. Lymph node biopsies by various invasive methods or not fully find their place in this type of association. The EBUS (endobronchial ultrasound) and EUS (endoscopic ultrasound) has a respective sensitivity of 78 and 89% in lymph node biopsies are superior to mediastinoscopy in the search for tumor localization [12]. Histological examination will show in the case of a CLL, lymphoid cells with a small homogeneous cytoplasm, weakly basophilic and devoid of nucleolus. The core has dark reinforcements clearly separated by lighter spaces, giving the impression of chromatin clods [13]. Our attitude was to attempt a posterolateral thoracotomy and mediastinal lymph node dissection first before any act of parenchymal resection. The negativity of dissection during a frozen section has enabled us to achieve a lobectomy. The prognosis of CLL depends on its stage as classified by Binet. The median survival of Stage C is 2 years while that of stage B resembles practically that of the general population [14]. For stage B as is the case in our patient, the median survival is 5 years. With this relatively poor prognosis like that of lung cancer that also relies the stage of the disease.
Thus the risk of cancer death in CLL patients is increased by 72% and deaths represent 64.8% in this population. Among these cancers, lung cancer and melanoma have a poor prognosis [5].
Ultimately, the CLL patients are known carriers of a risk of developing a second cancer. Several theories have attempted to explain this phenomenon, and particular attention should be paid to the role of chemotherapy. Lung cancer is part of this set of secondary cancers whose combination worsen further prognosis. A systematic search of these cancers should be attempted in order to establish early treatment strategies for improving the prognosis.
CONFLICT OF INTEREST
None declared
REFERENCES
1. Cazin B, Delmer A, Cymbalista F, Leblond V, Letestu R, Levy V, et al. Leucémie lymphoïde chronique. EMC – Hématologie 2013;8(3): 1–15.
2. Locatelli-Sanchez M, Couraud S, Souquet PJ. Epidémiologie du cancer bronchique : données actuelles. Rev Mal Respir Actual. 2015; 7: 285-289.
3. Mordant P, Pages PB, Foucault C, Badia A, Fabre E, Dujon A et al. Chirurgie des cancers bronchopulmonaires après traitement d’un premier cancer. Rev Mal Respir 2013; 30: 357-366.
4. Schollkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer 2007; 121: 151–156.
5. Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population - based study. British Journal of Cancer 2011;105: 1076 –81.
6. Hisada M, Biggar RJ, Greene MH, Fraumeni Jr JF, Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood 2001; 98: 1979–81.
7. Molica S. Second neoplasms in chronic lymphocytic leukemia: incidence and pathogenesis with emphasis on the role of different therapies. Leukemia & Lymphoma 2005; 46: 49–54.
8. Hsu CW, Krevsky B, Sigman LM, Thomas RM. Rapid progression of Barrett’s esophagus to metastatic esophageal carcinoma in a patient with chronic lymphocytic leukemia. J Clin Gastroenterol 1998; 27:261–4.
9. Benjamini O , Jain P , Trinh L, Qiao W, Strom SS, Lerner S et al. Second cancers in patients with Chronic Lymphocytic Leukemia who received frontline FCR therapy – Distribution and clinical outcomes. Leuk Lymphoma 2015; 56: 1643–1650.
10. Potti A, Ganti AK, Koch M, Mehdi SA, Levitt R. Identification of HER-2/neu overexpression and the clinical course of lung carcinoma in non-smokers with chronic lymphocytic leukemia. Lung Cancer 2001;34:227–232.
11. Berthelot C, Truchan-Graczyk M, Genevieve F, Poirier AL, Artur-Guillemette P, Vervueren L, et al. La tomographie par émissions de positons au 2-[18F] fluoro-2-désoxy-D-glucose (TEP-FDG) permet d’identifier les stades A et B des leucémies lymphoides chroniques (LLC). Médecine Nucléaire 2009; 33: 539-546.
12. Girard P, Caliandro R, Stern JB, Natali D, Lenoir S, Validire P, et al. Démarche diagnostique dans le cancer bronchique : diagnostic positif et bilan d’extension. Rev Mal Respir Actual. 2013 ; 5: 410-418.
13. Troussard X. Diagnostic, pronostic et traitement chez les patients avec une leucémie lymphoïde chronique. IBS. 2007; 22: 313-318.
14. Travade P. Leucemie Jympho'ide chronique. Rev Mect Interne 2000; 21: 108-11.
FIGURES
REFERENCES
1. Cazin B, Delmer A, Cymbalista F, Leblond V, Letestu R, Levy V, et al. Leucémie lymphoïde chronique. EMC – Hématologie 2013;8(3): 1–15.
2. Locatelli-Sanchez M, Couraud S, Souquet PJ. Epidémiologie du cancer bronchique : données actuelles. Rev Mal Respir Actual. 2015; 7: 285-289.
3. Mordant P, Pages PB, Foucault C, Badia A, Fabre E, Dujon A et al. Chirurgie des cancers bronchopulmonaires après traitement d’un premier cancer. Rev Mal Respir 2013; 30: 357-366.
4. Schollkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer 2007; 121: 151–156.
5. Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population - based study. British Journal of Cancer 2011;105: 1076 –81.
6. Hisada M, Biggar RJ, Greene MH, Fraumeni Jr JF, Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood 2001; 98: 1979–81.
7. Molica S. Second neoplasms in chronic lymphocytic leukemia: incidence and pathogenesis with emphasis on the role of different therapies. Leukemia & Lymphoma 2005; 46: 49–54.
8. Hsu CW, Krevsky B, Sigman LM, Thomas RM. Rapid progression of Barrett’s esophagus to metastatic esophageal carcinoma in a patient with chronic lymphocytic leukemia. J Clin Gastroenterol 1998; 27:261–4.
9. Benjamini O , Jain P , Trinh L, Qiao W, Strom SS, Lerner S et al. Second cancers in patients with Chronic Lymphocytic Leukemia who received frontline FCR therapy – Distribution and clinical outcomes. Leuk Lymphoma 2015; 56: 1643–1650.
10. Potti A, Ganti AK, Koch M, Mehdi SA, Levitt R. Identification of HER-2/neu overexpression and the clinical course of lung carcinoma in non-smokers with chronic lymphocytic leukemia. Lung Cancer 2001;34:227–232.
11. Berthelot C, Truchan-Graczyk M, Genevieve F, Poirier AL, Artur-Guillemette P, Vervueren L, et al. La tomographie par émissions de positons au 2-[18F] fluoro-2-désoxy-D-glucose (TEP-FDG) permet d’identifier les stades A et B des leucémies lymphoides chroniques (LLC). Médecine Nucléaire 2009; 33: 539-546.
12. Girard P, Caliandro R, Stern JB, Natali D, Lenoir S, Validire P, et al. Démarche diagnostique dans le cancer bronchique : diagnostic positif et bilan d’extension. Rev Mal Respir Actual. 2013 ; 5: 410-418.
13. Troussard X. Diagnostic, pronostic et traitement chez les patients avec une leucémie lymphoïde chronique. IBS. 2007; 22: 313-318.
14. Travade P. Leucemie Jympho'ide chronique. Rev Mect Interne 2000; 21: 108-11.
ARTICLE INFO
DOI: 10.12699/jfvpulm.9.26.2018.40
Conflict of Interest
Non
Date of manuscript receiving
21/02/2018
Date of publication after correction
16/4/2018
Article citation
Issoufou I, Belliraj L, Ammor F, Harmouchi H, Lakranbi M, Rachid S, Ouadnouni Y, Smahi M. Bronchopulmonary cancer and chronic lymphocytic leukemia: a unique combination for which prognosis? J Func Vent Pulm 2018;26(9):40-43.