
 English
English
 French
French
Medical treatment of obstructive sleep apnea in asthmatic children
Traitement médical des apnées obstructives du sommeil chez les enfants asthmatiques
Y. Nguyen Hoang1, S. Duong-Quy2,3, T. Nguyen-Thi-Dieu4
1: Department of Pediatrics. Phu Tho General Hospital, Phu Tho, Vietnam
2: Clinical Research Center. Lam Dong Medical College. Dalat, Vietnam
3: Division of Immuno-Allergology. Penn State Medical College. USA
4: Pediatric Department. Hanoi Medical University. Hanoi, Vietnam
Corresponding author
Dr. NGUYEN-THI-DIEU Thuy
Pediatric Department. Hanoi Medical University. Hanoi, Vietnam
E-mail: nguyendieuthuyhmu@gmail.com
ABSTRACT
Introduction. Obstructive sleep apnea (OSA) makes asthma more severe and difficult to control. Detection and treatment of OSA in patients with asthma significantly contributes to disease control.
Objectives. Combination Singular orally for 12 weeks in asthma prophylaxis may be more effective in treating apnea and better controlling asthma. Methods. This is a prospective descriptive study on 139 patients who were examined and treated for asthma. All the children were asked for the disease according to the study sample, measuring lung function, exhaled nitric oxide (NO), and respiratory polygraphy with Apnea-Link Plus before and after 3 months of treatment.
Results. The prevalence of OSA among asthmatic children was 71.2%, of which 61 (61.6%) were mild children (AHI 1-4/hour), 25 (25.3%) was moderate OSA (AHI 5-9/hour) and 13 (13.1%) with severe OSA (AHI ≥10/hour). Average AHI was 3.45±4.01/hour. The FENO was positively correlated with the decreased AHI. After 3 months of Singular prophylaxis, the patient responded well with an increase in ACT score (from 19.2 to 22.6 with P<0.05), improved lung function (FEV1
increased from 85.1% to 93.5%), associated with decreased AHI and improved night and daytime symptoms of sleep disorders.
Conclusion. Asthmatic children who have symptoms of snoring, irritated sleep, difficulty falling asleep or abnormal daytime behaviors should be screened for OSA. Prophylactic treatment with Singular for 12 weeks has been shown to reduce the AHI and better control asthma.
KEYWORDS: Asthma; Asthma control; Obstructive sleep apnea; Apnea-hypopnea index
RÉSUMÉ
Introduction. Les apnées obstructives du sommeil (AOS) rend l'asthme plus sévère et plus difficile à contrôler. La détection et le traitement de l'AOS chez les patients asthmatiques contribuent de manière significative au contrôle de la maladie.
Objectifs. La combinaison par voie orale pendant 12 semaines en prophylaxie contre l'asthme peut être plus efficace pour traiter AOS et mieux contrôler l'asthme.
Méthodes. Il s'agit d'une étude descriptive prospective sur 139 patients qui ont été examinés et traités pour l'asthme. Tous les patients ont été interrogés sur la maladie selon l'échantillon de l'étude, mesurant la fonction respiratoire, le monoxide d’azote (NO) expiré, et la polygraphie respiratoire avec Apnea-Link Plus avant et après 3 mois de traitement. IAH 1-4/heure), seulement 25 (25,3%) étaient AOS modérés (AHI 5- 9/ heure) et 13 (13,1% ) étaient graves (AHI ≥10/heure). IAH moyen: 3,45 ± 4,01/heure. FENO était corrélé positivement avec la diminution d’IAH. Après 3 mois de traitement Singulière; le patient a bien répondu à une augmentation du score ACT (de 19,2 à 22,6 avec P <0,05); une amélioration de la fonction respiratoire (FEV1: passé de 85,1% à 93,5%); IAH a été diminué, et les symptômes nocturnes et diurnes du sommeil
étaient améliorés.
Conclusion. Les patients atteints de l’asthme qui présentent des symptômes de ronflement, un sommeil agité, des difficultés à s'endormir ou qui ont des comportements diurnes anormaux doivent être soumis à un dépistage du AOS. Il a été dé-montré qu'un traitement prophylactique par Singular pendant 12 semaines réduit l'IAH et contrôle mieux l'asthme.
MOTS CLÉS: Asthme; Contrôle de l’asthme; Apnée obstructive du sommeil; Index d’apnées-hypopnées
INTRODUCTION
Obstructive sleep apnea (OSA) is a repeated successive partial or complete obstruction of the upper airway during sleep, resulting in a decrease in breathing or apnea completely despite respiratory effort [1,2]. OSA is the most common form of sleep disturbance, which is relatively common. In children, over the past few decades, OSA has been widely recognized as a significant cause of illness, accounting for 1% to 5% [2,3].
Asthma is a chronic inflammation of the airways with the participation of many inflammatory cells and inflammatory factors. OSA and asthma are two co-morbidities, both sharing symptoms because they are both related to airflow limitation and increased respiratory effort, as a result of airway obstruction during sleep [4]. In patients with asthma, OSA acts as a contributing mechanism to aggravating asthma because the decrease in airway in nocturnal asthma is associated with sleep distribution, difficulty sleeping, early waking. and daytime sleepiness [5].
Increased abdominal pressure during OSA contributes to gastroesophageal reflux (GER), increased bronchial responsiveness, and bronchitis [6]. Patients with uncontrolled asthma may experience an increase in the number of episodes of OSA and hypoxemia, especially during sleep with rapid eye movement [7]. OSA in asthmatics has been of interest and research in the last few years and has shown that OSA is comorbid and more common in asthmatics, since in these patients there is frequent obstruction and recovery of airways if asthma is not well controlled [8].
Montelukast is an effective, safe, well tolerated leukotriene receptor antagonist, and the US. Food and Drug Administration has approved it for the treatment of prophylaxis in childhood asthma and allergic rhinitis. from one year old up. Moreover, it does not cause resistance in long-term studies [9]. A single course of Singulair 4 mg daily for children under 6 years and Singulair 5 mg daily for children aged 6 years and older for 12 weeks is also effective in relieving and contributing to asthma control in asthma patients with OSA and uncontrolled asthma [2].
Therefore, we can understand the proportion of children with OSA asthma, the relationship between the severity of OSAS and the degree of control, the level of asthma helps to control well in the treatment of asthma. Therefore, we conducted this study with the objectives (1) to find out the asthma children at risk of OSA; (2) to learn the relationship between OSA severity and respiratory function and asthma control levels; (3) to confirm the effectiveness of OSA treatment in asthma children with Singular.
METHODS
Subjects
Pediatric patients diagnosed with asthma treated at the Department of Immunology - Allergy of National Children’s Hospital from December 2015 to the end of December 2017.
Criteria for selecting asthmatic children
Patients were diagnosed with asthma according to GINA 2015 criteria.
Patients without acute asthma attacks.
Patients and family members agree to participate in the study.
Exclusion criteria including one of the following
The patient was in an acute asthma attack.
Patients with facial deformities, nasal septum deformities, patients with hypertonic tonsils with surgical indications.
Patients and family members did not agree to participate in the study.
Diagnostic criteria for asthma
Applying the GINA (Global Initiative for Asthma) 2015 diagnostic criteria for children > 5 years-old for a definite diagnosis of asthma and asthma [10].
Methods
Study design
Objectives 1 and 2: Cross-sectional descriptive studies.
Objective 3: Study intervention interventions for asthma and OSA prophylaxis.
Study process
Patients who were eligible to be examined and selected for study group.
Classification of patients: according to the asthma stages 1 to 4 of GINA 2015 with the degree of asthma control. Asthma is fully controlled, partially, uncontrolled. ACT test - asthma control: family and patient self-assessment.
Measure FENO concentration, measure respiratory function
Diagnosis of OSA was based on the respiratory polygraphy: AHI in children: normal: AHI < 1/ hour, mild: AHI = 1 - 4 / hour, moderate: AHI = 5 - 9 / hour, severe: AHI ≥ 10 / hour [11].
Data collection
The disease and physical examination were recorded to determine previous preventive asthma, asthma level, level of asthma control. History of allergy in family: allergic rhinitis, allergic conjunctivitis, eczema, urticaria, drug allergy, food allergy, food, and bronchial asthma.
Laboratory tests
Respiratory polygraphy with ApneaLink Plus
Screening device Sleep Apnea at home, is a very simple, low cost, very easy to use with handheld device that can record up to 4 channels of accurate information: excessive breathing, heart rate , blood oxygen saturation, and airflow.
Respiratory polygraphy
Connect ApneaLink Plus to the computer and enter patient information; using ApneaLink for patients during the night at home; download the previous night data to the computer; printable report easily read including the following parameters: AHI: Apnea - hypopnea index: equals the total number of apnea or apnea (10s) multiplied by 60 (sleep time) and divides by the total sleep time.
ODI (Oxygen desaturation index): the total number of apnea and hypopnea with reduced oxygen saturation in the blood during one hour of sleep (regardless of the type of apnea).
OA (Obstructive apnea): stopped nasal - oral gas flow ≥ 10s with attempted ventilation when apnea.
CA (Central apnea): stopped nasal air flow ≥ 10s without attempted ventilation when apnea.
MA (Mixed Apnea): stopped nasal - oral gas flow ≥ 10s. Started as CA but ended with a ventilation attempt.
Other clinical tests
Blood count (count of white blood cells, leukocyte formula with an automatic device). Quantification of total IgE in blood. Respiratory function measurement: performed by the Koko meter at the Central Pediatric Respiratory
Functional Laboratory, recording the values of FVC, FEV1, FEV1 / FVC, PEF, MEF 25-75. Measuring NO concentration in exhaled gas: performed by NOBreath with 4 exhaled flow of 50,100,150,350 ml /sec, measuring NO nasal. Classify NO increase level according to ATS / ERS recommendation in children: FENO <20ppb: normal; 20-35 ppb: increase; >35ppb: elevation [8]. CaNO <4ppb, NO nasal ≥850 have diagnostic value of rhinitis [11].
Statistical analysis
processed on SPSS22.0 software. Qualitative variables are presented as numbers and percentages.
Quantitative variables are presented as mean and SD; check the normal or non-standard distribution of variables with the Skewness - Kurtosis test. Using Kruskal Wallis and H test for comparing median values FVC, FEV1, FEV1 / FVC. The relationship between AHI and respiratory function, FENO, CaNO by Spearman test.
Study ethics
This study did not cause any harm to patients and families. Patient and patient's family were explained firstly and voluntarily participated in the study. Patients who were not enrolled in the study were not discriminated against during treatment and monitoring. All information related to patients participating in the study was kept confidentially.
RESULTS
The incidence of obstructive sleep apnea syndrome (OSA) in patients with asthma is high, accounting for 71.2%. Of which 61.6% of asthma patients had mild OSA (AHI = 1 - 4), 25.3% had moderate OSA (AHI = 5 - 9) and 13.1% had severe OSA (AHI ≥ 10).
The average rate of apnea (AHI) was 3.45 ± 4.01 times.
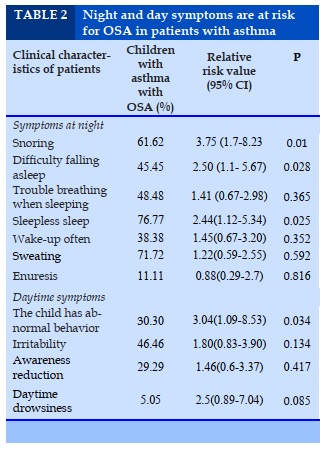
Characteristics of study subjects
There were 139 asthma patients participating in the study. Average age of study group was 9.3 yearsold. Male accounted for 73.4%; 88.5% had a history of allergy (eczema, allergic rhinitis, allergic conjunctivitis, urticaria, drug allergy, or food allergy) in which allergic rhinitis was the most common comorbidity: 83, 5%; 64.7% of families with parents, siblings or maternal grandparents with allergies and 12.2% with gastric reflux were diagnosed.
Most patients were diagnosed and treated for asthma for the first time, either given prophylaxis but infrequently or abandoned it. Only 12.9% of preventive treatment was followed the doctor's instructions.
The proportion of patients with asthma at stages 2 and 3 was quite high, accounting for 43.2% and 39.6%. Besides, the level of severe asthma in stage 4 accounts for 2.9%; stage 1 in asthma accounts for 14.4%. Children were exposed to cigarette smoke due to family smoking was by 44.7%.
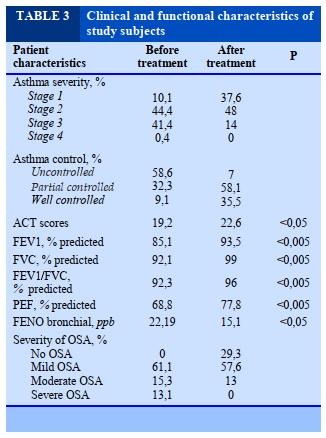
The symptoms of snoring in asthma patients with relative risk (RR) with OSA was 3.75 times higher than in patients with asthma without OSA (P = 0.01); symptoms of difficulty going to sleep and irritated sleep in patients with asthma was 2.50 and 2.44 times more likely to be at risk of OSA compared with patients with asthma without OSA (P = 0.028 and P = 0.025). Symptoms of sleep apnea, frequent waking, and perspiration sweating in patients with asthma increase the risk of OSA was not statistically significant (P >0.05). Symptoms of abnormal behavior in patients with asthma were at 3.04 times higher risk of having OSA compared with patients with asthma without OSA (P = 0.034). Symptoms of irritability, agitation, and daytime drowsiness in patients with asthma increased the risk of OSA without statistical significance (P >0.05).
Characteristics of patients with asthma with OSA after 3 months of treatment with Singular and asthma prevention
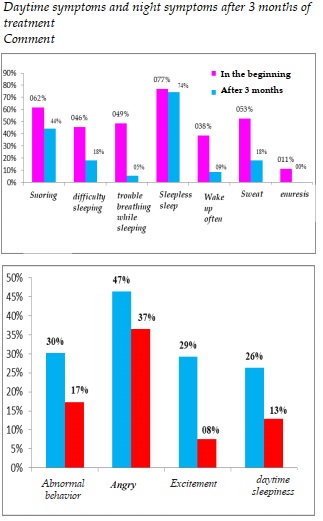
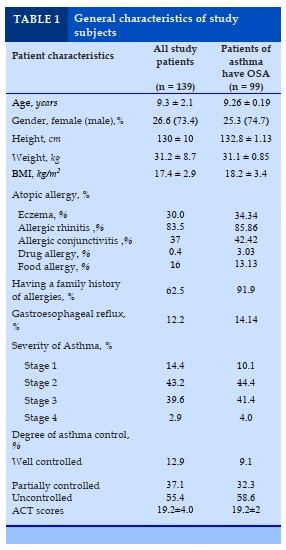
Daytime symptoms and night symptoms after 3 months of treatment
Comment
All symptoms during the day and night of sleep improved after 3 months of treatment, especially symptoms of sleep apnea decreased from 48.5% to 5.4%; snoring decreased from 61.6% to 44.1%; awake during sleep decreased from 38.4% to 8.6%.
The sweat at night decreased from 52.5% to 18.3%; bedwetting reduced from 11.1% to 0%. Day symptoms such as abnormal behavior of patients decreased from 30.3% to only 17.2%; patient agitation dropped from 29.3% to only 7.5%. The symptoms of daytime sleepiness decreased from 26.3% to 12.9%.
Clinical and functional characteristics after 3 months of treatment
After 3 months of treatment, the severity of asthma changed markedly at stage 1 to 37.6% compared to 10.1% at the beginning and stage 3 decreased from 41.4% to 14%. Particularly, stage 4 after 3 months of treatment is not exist (0.0%).
The degree of partial asthma control has increased from 32.3% to 58.1% and the level of complete asthma control has also increased significantly from 9.1% initially to 35.5%; ACT score also increased from 19.2 points to 22.6 points after 3 months of treatment with a statistically significant difference with P <0.05.
There was a marked improvement in respiratory function with all parameters on respiratory function increased, especially with the initial FEV1 of 85.1% and after 3 months of treatment. increased to 93.5%; peak flow increased from 68.8% at baseline to 77.8% after 3 months of treatment (P <0.005). The concentration of nitric oxide (FENO), bronchial inflammatory marker, gradually decreased from 22.19 ppb at the beginning to 15.1 ppb after 3 months of treatment with a statistically significant difference (P <0.05). After 3 months of treatment, 29.3% of asthma patients did not have OSA; the number of patients with asthma with OSA at baseline severity was 13.1% after 3 months of treatment, and no asthma patients had severe OSA. The average number of patients with asthma with OSA decreased from 25.3% to 13.0%.
DISCUSSION
Our study results demonstrated 139 children with asthma had the prevalence of OSA in asthma was 71.2% of which 61 (61.6%) was mild (AHI = 1-4/ hour); only 25 (25. 3%) moderate OSA (AHI = 5-9/hour) and 13 (13.1%) severity (AHI ≥10/hour).
Average AHI: 3.45 ± 4.01 times/ hour. Previously, OSA and asthma was considered to be the diagnosis of two separate diseases, but now some authors thought that they may actually be related. Indeed, OSA in asthmatics has been of interest and study in the last few years and has showed that OSA is comorbid and more common in asthmatic patients. A meta-analysis showed that the estimated prevalence of OSA in children with asthma was estimated at 63%; the risk of OSA and sleep disturbance in asthmatic patients was 3.7 and 1.7 times higher than those without asthma. The prevalence of OSA is higher in asthmatic patients, suggesting the existence of a relationship between OSA and asthma leads to a more severe clinical phenotype for both diseases [12].
Average age of study group was 9.3 years old. Male accounted for 72.8%. Most asthma patients with a history of allergy or in a family with allergies, in which allergic rhinitis was 85.86% the most common, is equivalent to the Fulvio Braid study: 80% of asthmatic patients with concomitant allergic rhinitis and rhinitis are associated with an increased risk of obstructive sleep apnea syndrome in patients with asthma [13]. When sleeping, the nasal cavity acts as the main route. Hence, inflammated nose causes mucosal congestion, edema, nasal stenosis inducing upper airway narrowing. Stimulation of chronic mucosal postero-rejection can lead to reflex bronchospasm through nasal-bronchial sympathetic reflex. Besides, replacing breathing with the mouth when the nose is obstructed, leading to the drying of air into the airways can cause asthma attacks.
Patients with asthma associated with OSA - rhinitis were higher than those without rhinitis. In 139 study patients, we encountered mostly mild and moderate asthma (43.2% and 39.6%). Meanwhile, mild intermittent asthma accounted for very low proportion of 14.4% and patients with severe asthma accounted for 2.9%. This may be due to the improvement the ability to diagnose, treat and control asthma at the early stage, but it may also be due to the low prevalence of uncontrolled and severe and difficult to treat asthma in children that accounts for very low proportion [14].
Regarding the level of asthma control, we also found that 55.4% of patients who had an acute asthma attack had not been very well controlled, many of whom had previously been on preventive treatment but had removed it voluntarily the treatment for a variety of reasons such as fear of drug side effects, self-recognition that the disease had been cured or was not fully awared of asthma prophylaxis; 31.7% of patients had asthma prophylaxis but not often. There are many similar clinical manifestations between OSA and asthma: reduced sleep quality at night, snoring, sleep apnea, apnea, restless sleep, frequent wake-ups, trouble falling asleep, sweating and enuresis. Other symptoms that result from OSA that occur during the day include: decreased concentration, decreased attention, impaired memory, mood changes (agitation, depression) [15]. In our study, asthma children had night symptoms such as snoring (61.6%), difficulty of falling asleep (45.5%), restless sleep (76.8%), waking up more frequent (34.8%), more common among children with OSA than those without OSA. On the other hand, snoring symptoms in patients with asthma are nearly four times more likely to have OSA than those without OSAS (P =0.01). This is a very important feature to help screen OSA in children with asthma and consistent with previous reports. Children who sleep at night snoring can be considered as a risk factor for OSA.
The present study also shows that some sleep symptoms are also at risk for OSA in children with
asthma, which is a sign that they have trouble falling asleep and not sleeping well. In these children, the relative risk of having OSA was 2.5 times higher than for children of asthma without OSAS. There was no statistically significant difference in daytime symptoms (abnormal behavior, irritability, agitation, daytime sleepiness) between the 2 groups of children. However, there was 5.05% of children with cognitive impairment in asthma children had severe OSA while other groups did not have this symptom. In children with severe OSA, daytime sleepiness has been shown to correlate with the severity of OSA. However, daytime drowsiness is less common in OSA children because children are often hyperactive [16].
The results of the study showed that after 3 months of treatment, the severity of asthma significantly changed at stage 1 to 37.6% compared to 10.1% at the beginning and stage 3 decreased from 41.4% to 14%. Particularly, stage 4 after 3 months of treatment is no longer presence. In addition, the level of asthma control has changed markedly as the level of partial asthma control has increased from 32.3% to 58.1% and the level of well asthma control has also increased significantly: from 9.1% at the beginning to 35.5%. The ACT score also increased from 19.2 points to 22.6 points after 3 months of treatment with a statistically significant difference with p <0.01. In our study, there was a marked improvement in respiratory function. All parameters of respiratory function evaluation increased, especially the initial FEV1 of 85.1%. and after 3 months of treatment increased to 93.5%. In addition, the FENO was also decreased significantly after 3 months of treatment from 22.19ppb at the beginning to 15.1ppb. It can be concluded that after 3 months of treatment, there was a significant improvement in airway dysfunction (increased FEV1) and a significant decrease in bronchial bio-marker. All symptoms at night and day of sleep improved after 3 months of treatment, especially symptoms of sleep apnea decreased from 48.5% to 5.4%. In addition, other OSA symptoms in children, such as snoring, were significant improved. Snoring and sleep apnea are the most common complaints of parents of children with OSA. Previous studies have reported these symptoms in more than 90% of cases [17]. However,
the history of snoring alone cannot distinguish between children with OSA and simple snoring in young children [18] and especially in children with asthma who has wheezing during sleep. Symptoms such as waking up to sleep, decreased sweating, and bedwetting all decreased significantly after 3 months. The symptoms of daytime drowsiness significantly improved after 3 months and decreased from 26.3% to 12.9%; Other daytime symptoms such as abnormal behavior of the patient decreased from 30.3% to only 17.2% and the patient's hyperactivity stimulation decreased from 29.3% to only 7.5%.
After 3 months of treatment with Singular, AHI was significantly improved. Our results also showed that after 3 months of treatment, up to 29.3% of children with asthma completely recovered from OSA, notably the number of asthma children with severe OSA at baseline was 13.1% after 3 months of treatment was reduced and to be in a lesser degree, and no asthma children with severe OSA syndrome. In addition, after 3 months of treatment, the mean number of asthma patients with OSA also decreased significantly. The study results confirmed that the treatment of asthma with ICS combined with antileucotriene drugs has the effect of controlling asthma, and has the effect of improving OSA.
CONCLUSION
The prevalence of OSAS among asthmatic children is 71.2%. Patients with asthma who are allergic or have a family history of allergies are at high risk for OSA. Patients with asthma who have symptoms of snoring, restless sleep, difficulty falling asleep or children who have abnormal daytime behaviors should be screened for OSAS. A 12-week Singular treatment with a combination of asthma prevention has been shown to reduce the incidence of obstructive sleep apnea and better control asthma.
CONFLICT OF INTEREST
Non
REFERENCES
1. Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. “Diagnosis and management of childhood obstructive sleep apnea syndrome”. Pediatrics. 2012 Sep;130(3):e714–e755.
2. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2014 Jan 17. “Montelukast for Sleep Apnea: A Review of the Clinical Effectiveness, Cost Effectiveness, and Guidelines”.
3. Lumeng JC, Chervin RD. “Epidemiology of pediatric obstructive sleep apnea”. Proc Am Thorac Soc. 2008 Feb;5(2):242–252.
4. Ekici, A., et al., Association of asthma-related symptoms with snoring and apnea and effect on health-related quality of life. Chest, 2005. 128(5): p. 3358-63.
5. Gutierrez, M.J., et al., Nocturnal phenotypical features of obstructive sleep apnea (OSA) in asthmatic children. Pediatr Pulmonol, 2013. 48(6): p. 592-600.
6. Shigemitsu, H. and K. Afshar, Nocturnal asthma. Curr Opin Pulm Med, 2007. 13(1): p. 49-55.
7. Lewis, D.A., Sleep in patients with asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med, 2001. 7(2): p. 105-12.
8. Cristina Salles, Regina Terse-Ramos. Obstructive sleep apnea and asthma.Jornal Brasileirode Pneumol. 2013. (39) 5.
9.https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020829s051_020830s052_021409s028lbl.pdf.
10. Global Initiative for athma. 2015. https://ginasthma.org/wp-content/uploads/2016/01/GINA_Report_2015_Aug11-1.pdf
11. Duong-Quy S, Hua-Huy T, Tran-Mai-Thi HT, Le-Dong NN, Craig TJ, Dinh-Xuan AT. Study of Exhaled Nitric Oxide in Subjects with Suspected Obstructive Sleep Apnea: A Pilot Study in Vietnam. Pulm Med. 2016;2016:3050918.
12. Nguyen-Hoang, Y., T. Nguyen-Thi-Dieu, and S. Duong-Quy, Study of the clinical and functional characteristics of asthmatic children with obstructive sleep apnea. J Asthma Allergy, 2017. 10: p. 285-292.
13. Fulvio Braido.“Sleep Apnea Risk in Subjects With Asthma With or Without Comorbid Rhinitis”. 2014 Dec;59(12):1851-6.
14. GINA – Severe Asthma and Difficult to Treat Asthma 2019.
15. Nguyen Thanh B. Study on clinical characteristics, sleep polygraphy and the effect of continuous positive airway pressure in the treatment of obstructive sleep apnea syndrome. PhD thesis, Hanoi Medical University, 2012.
16. Pereira, K.D., J.C. Roebuck, and L. Howell, The effect of body position on sleep apnea in children younger than 3 years. Arch Otolaryngol Head Neck Surg, 2005. 131 (11): p. 1014-6.
17. Brouilette, R., et al., A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr, 1984. 105(1): p. 10-4.
18. Carroll, J.L., et al., Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest, 1995. 108(3): p. 610-8.
FIGURE - TABLES
REFERENCES
1. Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. “Diagnosis and management of childhood obstructive sleep apnea syndrome”. Pediatrics. 2012 Sep;130(3):e714–e755.
2. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2014 Jan 17. “Montelukast for Sleep Apnea: A Review of the Clinical Effectiveness, Cost Effectiveness, and Guidelines”.
3. Lumeng JC, Chervin RD. “Epidemiology of pediatric obstructive sleep apnea”. Proc Am Thorac Soc. 2008 Feb;5(2):242–252.
4. Ekici, A., et al., Association of asthma-related symptoms with snoring and apnea and effect on health-related quality of life. Chest, 2005. 128(5): p. 3358-63.
5. Gutierrez, M.J., et al., Nocturnal phenotypical features of obstructive sleep apnea (OSA) in asthmatic children. Pediatr Pulmonol, 2013. 48(6): p. 592-600.
6. Shigemitsu, H. and K. Afshar, Nocturnal asthma. Curr Opin Pulm Med, 2007. 13(1): p. 49-55.
7. Lewis, D.A., Sleep in patients with asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med, 2001. 7(2): p. 105-12.
8. Cristina Salles, Regina Terse-Ramos. Obstructive sleep apnea and asthma.Jornal Brasileirode Pneumol. 2013. (39) 5.
9.https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020829s051_020830s052_021409s028lbl.pdf.
10. Global Initiative for athma. 2015. https://ginasthma.org/wp-content/uploads/2016/01/GINA_Report_2015_Aug11-1.pdf
11. Duong-Quy S, Hua-Huy T, Tran-Mai-Thi HT, Le-Dong NN, Craig TJ, Dinh-Xuan AT. Study of Exhaled Nitric Oxide in Subjects with Suspected Obstructive Sleep Apnea: A Pilot Study in Vietnam. Pulm Med. 2016;2016:3050918.
12. Nguyen-Hoang, Y., T. Nguyen-Thi-Dieu, and S. Duong-Quy, Study of the clinical and functional characteristics of asthmatic children with obstructive sleep apnea. J Asthma Allergy, 2017. 10: p. 285-292.
13. Fulvio Braido.“Sleep Apnea Risk in Subjects With Asthma With or Without Comorbid Rhinitis”. 2014 Dec;59(12):1851-6.
14. GINA – Severe Asthma and Difficult to Treat Asthma 2019.
15. Nguyen Thanh B. Study on clinical characteristics, sleep polygraphy and the effect of continuous positive airway pressure in the treatment of obstructive sleep apnea syndrome. PhD thesis, Hanoi Medical University, 2012.
16. Pereira, K.D., J.C. Roebuck, and L. Howell, The effect of body position on sleep apnea in children younger than 3 years. Arch Otolaryngol Head Neck Surg, 2005. 131 (11): p. 1014-6.
17. Brouilette, R., et al., A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr, 1984. 105(1): p. 10-4.
18. Carroll, J.L., et al., Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest, 1995. 108(3): p. 610-8.
ARTICLE INFO DOI: 10.12699/jfvpulm.11.33.2020.12 Conflict of Interest