
 English
English
 French
French
Mesothelioma in 2010
Mesothéliome en 2010
Y. Aelony
Clinical Professor, Harbor UCLA, Los Angeles, California - USA
Corresponding author
Pr Yossef AELONY
Clinical Professor, Harbor UCLA, Los Angeles, California - USA
E-mail: y.aelony@cox.net
ABSTRACT
Over 80% of malignant pleural mesothelium (MM) cases are linked to occupational exposure to croccidolyte asbestos.
The natural history of MM is quite variable, although many studies suggest an average of 6-12 months survival. A study of untreated MM, however, showed a 5 year survival of 8%. Most cases present with a unilateral pleural effusion with a lymphocytic predominant exudate. Other cases may present with localized or diffuse pleural masses.
Medical thoracoscopic talc poudrage under local anesthesia (TTP) resulted in a 19-month median survival (mean 24 months) for consecutive MM patients presenting with pleural effusion. Medical thoracoscopic talc poudrage under local anesthesia (TTP) resulted in a 19-month median survival (mean 24 months) for consecutive MM patients presenting with pleural effusion. Chemotherapy with platins and antifolates improved survival by over 2 months compared to platins alone, but quality of life declined during therapy.
Optimal management of this uncurable disease remains uncertain, in the absence of controlled studies.
KEYWORDS: asbestos, mesothelium, malignant pleural mesothelium, pleural effusion, talc poudrage
RÉSUMÉ
Plus de 80% des cas de mésothéliomes pleuraux malins (MM) sont liés à l‘exposition professionnelle à l‘amiante. L‘incidence du MM continuera à s‘accroître au Viet Nam dans les 20 à 30 ans depuis l‘importation et l‘usage de l‘amiante, en particulier dans la construction et la contruction navale, au cours des dernières décennies en Asie.
L‘histoire naturelle du MM est assez variable, malgré de nombreuses études suggérant une survie moyenne de 6 à 12 mois. Néanmoins, une étude sur les MM non traités a montré un taux de survie à 5 ans estimé à 8%. De nombreux cas de MM se présentent avec une pleurésie unilatérale exsudative à prédominance lymphocytaire, d‘autres avec des masses pleurales localisées ou diffuses.
Le talcage pleural sous anesthésie locale aboutit à une médiane de survie de 19 mois (moyenne de 24 mois) pour des patients consécutifs présentant un épanchement pleural. La chimiothérapie à base de platines et d‘antifolates a prolongé la durée de survie de 2mois comparé aux platines seules, mais la qualité de vie a diminué au cours de la cure.
La prise en charge optimale de cette maladie incurable demeure incertaine en l‘absence d‘études contrôlées.
MOTS CLES: asbestose, mésothéliome, mésothéliome pleural malin, épanchement pleural, talcage pleural
INTRODUCTION
Malignant pleural mesothelioma is a relatively rare malignancy, affecting only 2000 to 3000 patients in the United States in recent years. Its importance is augmented, however, by the inadequacy of effective treatments, which so far have resulted in no apparent cures. Public interest has focused on its causation, since approximately 80% of cases are related to occupational exposure to amphibole asbestos (usually ‘blue’ asbestos known as crocidolite). This product was widely used in the 20th century for insulation in the shipping industries, in pipefitting, in production of automobile brakes, mixed in cement, and in many other applications. Although use of amphibole asbestos has been curtailed in recent decades, the delay in manifestation of disease for 20 to 60 years has lead to an increasing incidence. This is especially true in Europe, where the incidence of mesothelioma is not expected to peak for another ten years. One estimate suggests 1% of all European deaths in males born in the 1940s will be associated with mesothelioma in 2020. In Asia, a similar peak incidence in 2020 may be surmised. However, the diagnosis of this pleural-based disease requires an informed physician community and highly specialized pathologists. In countries where smoking is widespread, lung cancer is much more common and the less-familiar mesothelioma is underdiagnosed. In Vietnam, where new-onset tuberculosis (TB) occurs in 150,000 people annually, malignant pleural mesothelioma would seem less important. The World Health Organization has estimated that perhaps 775 cases annually of mesothelioma ought to be found in Vietnam. While this number is relatively small, nearly all cases die within a few years, whereas most of those afflicted with tuberculosis, for instance, will survive, if given appropriate therapy. The 20% of mesothelioma patients who deny any asbestos exposure presumably have been exposed to other fibers. In addition, high dose irradiation can induce mesothelioma after many years and genetic factors also play a role in pathogenesis of the disease. It is believed (& hoped) that chrysotile asbestos, which is still massively mined, does not cause mesothelioma when inhaled.
CLINICAL PRESENTATION AND PATHOLOGY
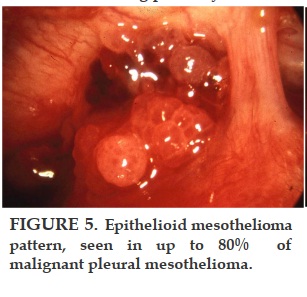
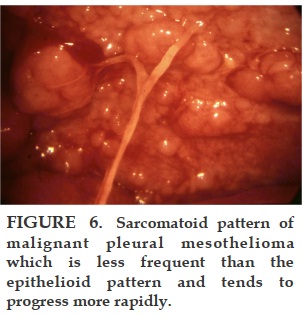
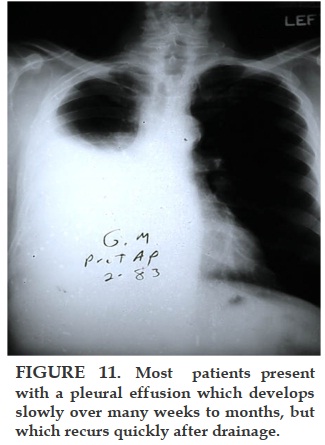
Most patients with malignant pleural mesothelioma will present with shortness of breath, due to the presence of a unilateral exudative, lymphocytic-predominant pleural effusion, which usually recurs if removed by thoracentesis. As the disease advances, the patients present with no effusion but with pleural masses. Biopsy of the parietal pleura with a closed biopsy needle (Cope or Abrams) or under direct vision through the thoracoscope reveals an epithelial pattern in about 80% of the patients. The remainder have a spindle cell or fibrosarcomatous pattern, which may be mixed with epithelioid cells. The greater the presence of fibrosarcomatous cells, the worse the prognosis. The diagnosis of malignant pleural mesothelioma has been difficult even for experienced pathologists, so we have sent all of our suspicious specimens to an international committee of experts for review.
NATURAL HISTORY
An international survey of malignant pleural mesothelioma in 1982 revealed that the median survival after diagnosis was only 6-12 months [1]. This figure has been used by some investigators to prove that their patients benefited from various treatments, whenever they lived longer than 12 months. This has thus far led to false conclusions, since, as in many tumors, there are wide variations in the aggressiveness of the disease. The wise investigator selects the best early-stage candidates for aggressive treatment. When these patients live longer than 12 months, investigators falsely conclude that the ‘prolonged survival’ is due to the treatment rather than the natural course of the disease. The only way to avoid this selection bias is to randomize patients into treatment and non-treatment groups for comparison, which has rarely been done in this disease. Rather than assuming a 6-12 month survival, one has to look to a study by Law in 1984 that found that 8% of untreated patients lived for 5 years [2]. One of these patients lived for 16 years with ‘no treatment’. Thus, controlled phase III studies are necessary to establish benefits for a given treatment.
DIAGNOSIS
Diagnosis in the past was largely made by thoracocotomy. However, in the 21st century clinical suspicion and biopsy by less invasive means have taken precedence, and thoracotomy should be reserved for research centers that are trying new modalities of therapy. A major search is underway for serum markers of early mesothelioma that would permit earlier detection and treatment of the disease. So far Epithelial membrane antigen (EMA), Mesothellin, Osteopontin, & Podoplanin have shown promise but have sensitivities and specificities less than 80% [3]. Today, closed or thoracoscopic parietal pleural biopsy are the mainstay of diagnosis.
STAGING OF DISEASE
Staging is very important in advising the patient of his immediate prognosis and in making prelimary comparisons between the results of published studies. It is also used to assess patients for aggressive surgical therapy. Staging has been unsatisfactory partly because the natural tendency in the last century was to assume that malignant pleural mesothelioma acts like lung cancer. However, since this disease arises in the parietal pleura not the lung, its progression is different from lung cancer, which begins in the parenchyma and moves sequentially to the locoregional lymph nodes and then metastasizes to the mediastinum and throughout the body. Contrarily, mesothelioma in the parietal pleura drains directly to the mediastinum nodes via lymphatics, perhaps explaining why mesothelioma has not been cured by early detection: the tumor appears to spread widely via lymphatics prior to any symptoms, thus prior to diagnosis and treatment.
When the tumor is grossly confined to the parietal pleura, the prognosis is better as it is earlier in the course of the illness. This is stage Ia by the International Mesothelioma Interest Group (IMIG) protocol [4]. Survival is shorter as soon as it invades the visceral pleura, (stage Ib) or the lung (stage II). Visible unilateral lymph node involvement in the mediastinum or invasion of the chest wall, pericardium, or diaphragmatic muscle is stage III, with a still worse prognosis. Aggressive surgeons argue that they can remove all the visible tumor at this stage, although until now it has always recurred afterwards. Stage IV includes contralateral involvement, distant metastatic disease, or whenever extensive disease cannot be grossly removed surgically. The usual staging procedures in lung cancer have proved misleading in malignant pleural mesothelioma.
CT scans miss 16% of nodes seen at mediastinoscopy. Mediastinoscopy failed to find + lymph nodes which were seen at thoracotomy later in 2/3 of cases [5]. So thoracotomy seems to be necessary for accurate staging. But until thoracotomy can be shown to have a survival benefit, it may be inhumane to do a thoracotomy at this time, with the possible exception being as part of a prospective randomized phase III therapeutic trial. Prognostication (i.e. staging) is currently being refined in nonsurgical groups in chemotherapy trials [6-8]. Curran et al. [6] showed a statistically significant difference in survival using the European Organization of Research and Treatment of Cancer criteria: negative prognostic factors included a white blood cell count > 8.3 × 103/ml , Eastern Cooperative Oncology Group performance status ≥1, sarcomatoid tumor cell type, male sex, and ‘probable’ versus ‘possible’ histologic diagnosis versus definite diagnosis. Three or more of these factors defined the high-risk group. Median survival was 10.8 months in the low-risk group and 5.5 months in the high-risk group (p = 0.0001). Similar findings were detected using the Cancer and Leukemia Group B staging scheme, in which patients were grouped by similar prognostic factors, including performance status, chest pain, dyspnea, platelet count > 400,000/μl, weight loss, serum lactate dehydrogenase level > 500 IU/l, pleural involvement, low hemoglobin level, high white blood cell count, and age > 75 years (versus age < 49 years) [8].
A preliminary study on positron emission tomography (PET) scanning reports that the intensity of 18F-fluoro-deoxyglucose uptake was superior to histological grade (well-differentiated versus poorly differentiated tumor) in predicting survival [9]. Moreover, the distribution of uptake correlated well with tumor burden observed at surgery. The importance of PET scanning when considering extrapleural pneumonectomy was also emphasized, because presence of extrathoracic disease contraindicates a surgical attempt at cure.
Recently, tumor necrosis – which correlated with angiogenesis – was shown to be an independent indicator of poor prognosis [10]. Whereas the median survival was 8.3 months for patients whose specimens failed to show tumor necrosis, median survival was 5.3 months for patients whose biopsy did show tumor necrosis (p = 0.008). The difference was even greater with epithelial mesothelioma.
We recently demonstrated that pleural pH is an important staging modality in malignant epithelial mesothelioma, with pH above 7.32 predicting twice the mean survival rate as pH < 7.32 (13 versus 26 months) [11]. The pleural pH must be quickly determined with the same accuracy as arterial pH, using calibrated blood gas machines. Use of urine test tapes for pleural pH has been shown to be too insensitive for this use.
Unfortunately, no one yet has sought to stage patients prospectively using all of the above noninvasive modalities.
TREATMENT
Many options have been tried with variable survival [12].
1. ‘No treatment’ or palliation only was associated with 18 month survival after onset of symptoms and 8% of 5 year survival [2].
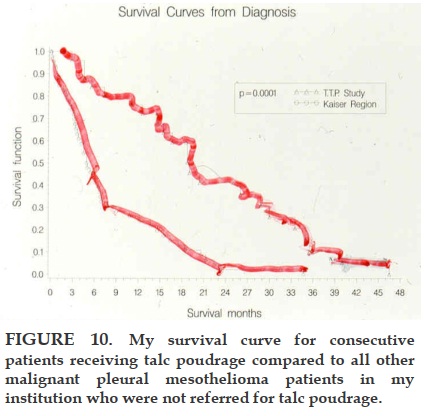
2. Thoracoscopic Talc Pleurodesis: up to 19 month median survival [13].
3. Thoracoscopic Talc Pleurodesis in stage Ia disease : 33 months median survival [14].
4.Pleurectomy in stage I disease: 34 month survival [15].
5. Pneumonectomy, radiation therapy, and chemotherapy have been tried separately or as combined ‘trimodality’ treatment, with variable results [12,16].
6. “Trimodality”: 1% of 5 year survival if all patients evaluated are included in the denominator [16].
As indicated previously, all studies are difficult to generalize due to conscious or unpreventable selection of patients. Many studies do not compare their survivals to the survival of the patients who were refused entry into the study. Palliative treatments have been recommended as being most humane, since virtually no cures have occurred. Hospice care has greatly helped patients and most important the patient needs to be followed by a physician team who are both well informed about the disease and sympathetic to patient complaints [12].
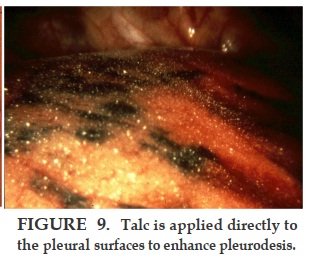
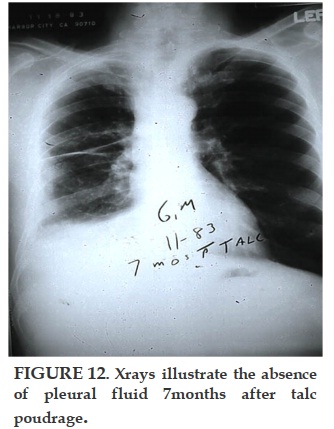
Thoracoscopic talc poudrage (pleuroscopy under local anesthesia) has been shown to have a 90-100% effectiveness in preventing recurrence of pleural effusion for months to years afterwards [13, 14]. Survival is related to the stage of disease [11, 14]. There is no proven anti-neoplastic effect of talc, but it clearly is the most effective pleurodesic agent studied so far in malignant pleural mesothelioma. Research scientists have shown apoptosis effects of talc in vitro, but these studies have so far not been accepted as relevant to the human disease [17]. Indwelling catheters to drain pleural effusion have been used in lieu of talc pleurodesis, especially in advanced disease with a short life-expectancy. Published data shows that this is an option even for patients who are not yet in a terminal condition, but the mesothelioma survivals published are much shorter than those treated with talc poudrage; furthermore, those who spontaneously pleurodesed during long-term catheter drainage lived 3.5 times longer than those who did not pleurodese, suggesting that pleurodesis itself may prolong life in malignant effusions [18, 19]. Reports of mesothelioma pleurodesis with agents other than talc are so rare that presumably they have been tried and failed. Tetracycline specifically has been shown to be ineffective in the pleural pH range of most mesotheliomas [20].
Decortication of visceral pleura and parietal pleurectomy has been described in early stage mesothelioma [15]. This has a lower morbidity and mortality than extrapleural pneumonectomy.
The 34-month survival was ascribed to the surgical treatment, but this was not significantly better than simple thoracoscopic talc poudrage in stage Ia patients-33 months [14]. Both studies confirm that limiting patients to those with early stage disease results in a longer survival after the procedure. The staging at thoracotomy is more accurate than at thoracoscopy, so it would not be possible to conclude that the two approaches are equally effective without doing a prospective randomized study.
Radiation therapy (XRT) is very effective at suppressing mesothelioma outgrowths to the chest wall along the needle track of prior thoracentesis or a surgical procedure, but is ineffective in controlling the internal disease because it is very widespread already at the time of presentation. Some centers offer 4000 rads of prophylactic irradiation to any needle or surgical tract in mesothelioma, but we found only 1 outgrowth in 40 mesothelioma thoracoscopies (unpublished data), so we reserve XRT for the occasional symptomatic outgrowth.
Chemotherapy has been very disappointing with responses of less than 25%. Recently, combining Pemetrexed (& other anti-folates) with cis-platin (& other platins) has been endorsed by oncologists who found that those taking both drugs lived about 2 months longer than those who took only cis-platin [21]. However, the few quality of life studies published with these drugs & their congeners suggest that most patients experience fatigue, 15 % have nausea & vomiting, & a significant number have hematological & other life-threatening side effects. All but one study failed to systemically control pleural effusions with talc poudrage; a major benefit of most studies was relief of dyspnea, which could have been controlled by the non-toxic talc poudrage. The failure to do pleurodesis prior to chemotherapy biases nearly all the studies reported. We sorely need prospective randomized studies of talc poudrage versus combined chemotherapy.
Extrapleural pneumonectomy and thoracic surgery in general have made tremendous strides in the past 30 years, reducing 30 day mortality from about 30% to only 5-10% in most recent studies, from the centers where this kind of aggressive therapy is performed on a regular basis [16]. This surgery requires reconstruction of the diaphram and partial removal of the pericardium. The most experienced surgical team, at the Harvard Medical School in Boston still reported in 2010 major morbidity in 35% of patients, 12% vocal cord paresis, 5% tracheostomy, re-operation for bleeding in 4%, empyema 5% [22]. Other studies have noted < 10% reoperations for rupture of the repaired diaphragm [23]. In the past, frequent suicide in operated patients was reported but recent data is unavailable.
The survival data are difficult to credit in the large 1999 study because there was only a mean 13 month followup [16]. The published long-term survivals were statistical estimates rather than true values. They reported 50% 5 year survival in early stage disease, those with < 1cc of residual tumor after surgery. But their ‘50%’ represented only 7 of 14 patients followed for 5 years. The discussion of the paper brought out that they interviewed between 700 and 800 mesothelioma patients in order to select about 183 patients for surgery.
Thus the 7 patients actually alive after 5 years only represented 1% of the total, compared to 8% in ‘untreated’ patients [2]. The former patients had ‘trimodality’ therapy and it is impossible to allocate which, if any, of the 3 modalities was most important.
The survival data are difficult to credit in the large 1999 study because there was only a mean 13 month followup [16]. The published long-term survivals were statistical estimates rather than true values. They reported 50% 5 year survival in early stage disease, those with < 1cc of residual tumor after surgery. But their ‘50%’ represented only 7 of 14 patients followed for 5 years. The discussion of the paper brought out that they interviewed between 700 and 800 mesothelioma patients in order to select about 183 patients for surgery. Thus the 7 patients actually alive after 5 years only represented 1% of the total, compared to 8% in ‘untreated’ patients [2]. The former patients had ‘trimodality’ therapy and it is impossible to allocate which, if any, of the 3 modalities was most important.
After 11 years, the actual survival data on the cohort of 183 operations has not been reported. A prospective study comparing surgical and non-surgical treatments has been initiated in the United Kingdom, and may be reported in 2011. Simple debulking of mesothelioma surgically to increase effectiveness of chemotherapy seems logical but has not been shown to prolong survival or improve quality of life.
Gene therapy and interferon gamma have shown promise in a few examples of very early disease, but research is still in its infancy. I had a patient who was asymptomatic while working fulltime in construction for 4 years after talc poudrage alone. Undoubtedly, he had a slow growing process. Clearly some mesotheliomas are slow growing, so a handful of selected patients who have longer survivals after treatment must be considered as only possible beneficiaries of the treatment.
CONCLUSION
In conclusion, we have shown that survival is short, but variable in this unfortunate disease process. Diagnosis requires a high index of suspicion and sophisticated pathological analysis. Some patients have an indolent form of the disease and live for several years, even without treatment. Treatment considerations should be focused on symptoms, with thoracoscopic talc poudrage creating a demonstrated benefit in quality of life, by relieving dyspnea.
Aggressive treatment with surgery, irradiation, and chemotherapy should ideally be limited to research centers doing prospective studies, since the putative benefits are uncertain and information is not available to completely allow balancing of side effects and hazards of treatment (as in immediate death from surgery or chemotherapy complications) with quality of life and length of survival. Hopefully future phase III studies will demonstrate some beneficial treatments of this disease.
In both industrialized countries and countries with emerging economies, scarce monetary resources should be funneled to research centers, to humane hospice care programs, and to measures that further limit exposure to amphibole asbestos.
CONFLICT OF INTEREST
No conflict of interest.
REFERENCES
1. Hillardal G. Malignant Mesothelioma 1982: review of 4710 published cases. Br J Dis Chest 1983; 77: 321.
2. M R Law, A Gregor, M E Hodson, H J Bloom, and M Turner-Warwick Malignant mesothelioma of the pleura: a study of 52 treated and 64 untreated patients. Thorax 1984; 39(4): 255–259.
3. Robinson BWS et al. Mesothellin-family proteins and diagnosis of mesothelioma. Lancet 2003; 362:1612-1616.
4. Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995; 108: 1122–1128.
5. Rice et al. Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg 2005; 80: 1988-93
6. Curran D et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer Experience. J Clin Oncol 1998; 16: 145–152.
7. Herndon JE, Green MR, Chahinian AP, Corson JM, Suzuki Y, Vogelzang NJ: Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998; 113: 723–731.
8. Edwards JG, Abrams KR, Leverment JN, Spyt TJ, Waller DA, O’Byrne KJ: Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax 2000; 55: 731–735.
9. Gerbaudo VH, Britz-Cunningham S, Sugarbaker DJ, Treves ST: Metabolic significance of the pattern, intensity and kinetics of 18F-FDG uptake in malignant pleural mesothelioma. Thorax 2003; 58: 1077–1082.
10. Edwards JG, Swinson DE, Jones JL, Muller S, Waller DA, O’Byrne KJ: Tumor necrosis correlates with angiogenesis and is a predictor of poor prognosis in malignant mesothelioma. Chest 2003; 124: 1916–1923.
11. Aelony Y, Yao J, King R. 'Prognostic Value of Pleural Fluid pH in Epithelial Malignant Mesothelioma after Talc Poudrage. Respiration 2006; 73: 334-339.
12. Aelony Y: Treatment of mesotheliomatous pleural effusion: experimental therapy versus thoracoscopic talc poudrage? Pro: talc poudrage therapy. J Bronchol 2001; 8: 54–59.
13. Aelony Y, Yao J. Prolonged survival after talc poudrage for malignant pleural mesothelioma: case series. Respirology 2005; 10: 649-655.
14. Boutin C et al. Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 1993; 72/2; 394-404.
15. Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999; 1799-804.
16. Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, et al: Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999; 117: 54–63; discussion 63–65
17. Najmunnisa N, Mohammed KA, Brown S, et al. Talc mediates angiostasis in maligant pleural effusions via endostatin induction. Eur Respir J 2007; 29: 761-769.
18. Tremblay A, Michaud G. Single-center experience with 250 tunneled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129: 362-368.
19. Aelony Y. Tunneled pleural catheters in malignant pleural effusion (letter). Lancet 2007: 370; 387.
20. Sahn SA, Good JT Jr: Pleural fluid pH in malignant effusions. Diagnostic, prognostic, and therapeutic implications. Ann Intern Med 1988; 108: 345–349.
21. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al: Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–2644.
22. Wolf et al. characteristics of malignant pleural mesothelioma in women. Ann thorac Surg 2010; 90: 949-56.
23. Byrne JG, Karavas AN, Colson YL, Bueno R, Richards WG, Sugarbaker DJ, Goldhaber SZ. Cardiac decortication (epicardiectomy) for occult constrictive cardiac physiology after left extrapleural pneumonectomy. Chest 2002; 122(6): 2256-9.
FIGURES
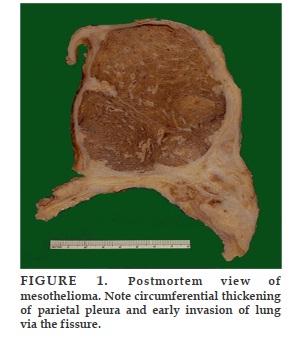
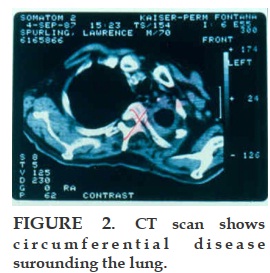
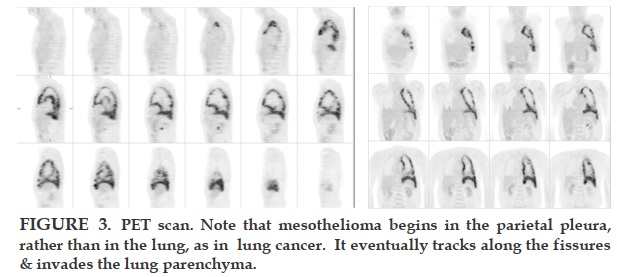
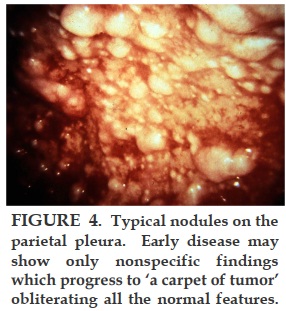
REFERENCES
1. Hillardal G. Malignant Mesothelioma 1982: review of 4710 published cases. Br J Dis Chest 1983; 77: 321.
2. M R Law, A Gregor, M E Hodson, H J Bloom, and M Turner-Warwick Malignant mesothelioma of the pleura: a study of 52 treated and 64 untreated patients. Thorax 1984; 39(4): 255–259.
3. Robinson BWS et al. Mesothellin-family proteins and diagnosis of mesothelioma. Lancet 2003; 362:1612-1616.
4. Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995; 108: 1122–1128.
5. Rice et al. Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg 2005; 80: 1988-93
6. Curran D et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer Experience. J Clin Oncol 1998; 16: 145–152.
7. Herndon JE, Green MR, Chahinian AP, Corson JM, Suzuki Y, Vogelzang NJ: Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998; 113: 723–731.
8. Edwards JG, Abrams KR, Leverment JN, Spyt TJ, Waller DA, O’Byrne KJ: Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax 2000; 55: 731–735.
9. Gerbaudo VH, Britz-Cunningham S, Sugarbaker DJ, Treves ST: Metabolic significance of the pattern, intensity and kinetics of 18F-FDG uptake in malignant pleural mesothelioma. Thorax 2003; 58: 1077–1082.
10. Edwards JG, Swinson DE, Jones JL, Muller S, Waller DA, O’Byrne KJ: Tumor necrosis correlates with angiogenesis and is a predictor of poor prognosis in malignant mesothelioma. Chest 2003; 124: 1916–1923.
11. Aelony Y, Yao J, King R. 'Prognostic Value of Pleural Fluid pH in Epithelial Malignant Mesothelioma after Talc Poudrage. Respiration 2006; 73: 334-339.
12. Aelony Y: Treatment of mesotheliomatous pleural effusion: experimental therapy versus thoracoscopic talc poudrage? Pro: talc poudrage therapy. J Bronchol 2001; 8: 54–59.
13. Aelony Y, Yao J. Prolonged survival after talc poudrage for malignant pleural mesothelioma: case series. Respirology 2005; 10: 649-655.
14. Boutin C et al. Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 1993; 72/2; 394-404.
15. Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999; 1799-804.
16. Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, et al: Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999; 117: 54–63; discussion 63–65
17. Najmunnisa N, Mohammed KA, Brown S, et al. Talc mediates angiostasis in maligant pleural effusions via endostatin induction. Eur Respir J 2007; 29: 761-769.
18. Tremblay A, Michaud G. Single-center experience with 250 tunneled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129: 362-368.
19. Aelony Y. Tunneled pleural catheters in malignant pleural effusion (letter). Lancet 2007: 370; 387.
20. Sahn SA, Good JT Jr: Pleural fluid pH in malignant effusions. Diagnostic, prognostic, and therapeutic implications. Ann Intern Med 1988; 108: 345–349.
21. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al: Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–2644.
22. Wolf et al. characteristics of malignant pleural mesothelioma in women. Ann thorac Surg 2010; 90: 949-56.
23. Byrne JG, Karavas AN, Colson YL, Bueno R, Richards WG, Sugarbaker DJ, Goldhaber SZ. Cardiac decortication (epicardiectomy) for occult constrictive cardiac physiology after left extrapleural pneumonectomy. Chest 2002; 122(6): 2256-9.
ARTICLE INFO
DOI: 10.12699/jfvp.2.2.2011.21
Conflict of Interest
Non
Date of manuscript receiving
12/9/2010
Date of publication after correction
15/01/2011
Article citation
Aelony Y. Mesothéliome en 2010. J Func Vent Pulm 2011;02(02):21-27.