
 English
English
 French
French
Study of correlation between exhaled NO measured by portable device and bronchial hyperresponsiveness in subjects with chronic cough
Etude de la corrélation entre NO exhale, measuré par l’appareil portable et l’hyperréactivité bronchique chez les sujets ayant la toux chronique
Q. Pham-Huy1, T. Tran-Ngoc2, T. Nguyen-Van2, B. Nguyen-Quoc2, K. Le-Quang2, D. Tran-Thanh2, N. Doan-Thi-Quynh2, T. Craig3, S. Duong-Quy2,3
1 : Hai Phong University of Medicine and Pharmacy
2 : Lam Dong Medical College
3: Penn State Medical College
3 : Service de Radiologie du CHU- Yalgado Ouédraogo, Ouagadougou, Burkina Faso
Corresponding author
Dr. Sy DUONG-QUY
Lam Dong Medical College, Dalat-VN. Penn State Medical College. USA
Email: sduongquy.jfvp@gmail.com
ABSTRACT
Introduction. Measurement of nitric oxide (NO) in exhaled breath may help to diagnose asthmatic patients have nocturnal chronic cough as a main symptom. However, the correlation between the level of fractional exhaled NO (FENO) and bronchial hyperresponsiveness (BHR) in asthma has not been clearly demonstrated.
Objective. This study aimed to evaluate the level of FENO and BHR in subjects with chronic cough, the correlation between the level of FENO and concentration of PD20 – methacholine, and the cut-off of FENO for diagnosis of BHR. Method. Subjects with chronic cough were included in this study. They underwent FENO measurements, methacholine challenge and lung functional testing.
Results. There were 32 healthy control subjects and 67 subjects with chronic cough were included in this study. The mean age and male - female ratio were not different between two groups (43±7 vs 42±6 years; 1.4 vs 1.3). The level of FENO in subjects with chronic cough was significant higher than that in control subjects (37±12 vs 15±8 ppb; P <0.01). The percentage of subjects had the positive BHR in subjects with chronic cough was significantly higher control subjects (55.2 vs 3.1 %; P<0.001). In subjects with BHR (+), FENO level was tightly correlated with log PD20 (R = -0.83; P<0.001). The cut-off of FENO level for diagnosis of BHR (+) is >36 ppb with the highest sensitivity and specificity (>80% and >75%).
Conclusion. The measurement of FENO with portable device is a useful tool to diagnose subjects with bronchial hyperresponsiveness, especially for subjects with chronic cough.
KEYWORDS: Chronic cough; nitric oxide; FENO; bronchial hyperresponsiveness.
RÉSUMÉ
Introduction. La mesure de monoxide d’azote (NO) dans l'air expirée peut aider à diagnostiquer les patients asthmatiques présentent une toux chronique nocturne comme le symptôme principal. Cependant, la corrélation entre le niveau de NO exhalé fractionné (FENO) et l'hyper-réactivité bronchique (HRB) n'a pas été clairement démontrée.
Objectif. Cette étude visait à évaluer le niveau de FENO et de HRB chez les sujets ayant de toux chronique, la corrélation entre le niveau de FENO et la concentration de PD20 - méthacholine, et le seuil de FENO pour le diagnostic de HRB.
Méthode. Les sujets atteints de toux chronique ont été inclus dans cette étude. Ils ont bénéficié des mesures de FENO, des tests de provocation à la méthacholine et des tests fonctionnels respiratoires. Résultats. Il y avait 32 sujets témoins sains et 67 sujets avec toux chronique ont été inclus dans cette étude. L'âge moyen et le rapport hommes-femmes n'étaient pas différents entre deux groupes (43±7 vs 42±6 ans; 1,4 vs 1,3). Le taux de FENO chez les sujets présentant une toux chronique était significativement supérieur à celui observé chez les sujets témoins (37±12 vs 15±8 ppb; P<0,01). Le pourcentage de sujets ayant un HRB positif chez les sujets présentant une toux chronique était significativement plus élevé chez les sujets témoins (55,2 vs 3,1%; P<0,001). Chez les sujets ayant d'HRB (+), le taux de FENO était étroitement lié au Log PD20 (R =-0,83; P<0,001). Le seuil de FENO pour le diagnostic de la HRB (+) est > 36 ppb avec la sensibilité et la spécificité les plus élevées (> 80% et> 75%).
Conclusion. La mesure de FENO avec un appareil portable est un outil utile pour diagnostiquer les sujets présentant une hyperréactivité bronchique, en particulier chez les sujets présentant une toux chronique.
MOTS CLÉS: : Toux chronique; monoxide d’azote; FENO; hyper-réactivité bronchique.
INTRODUCTION
Nitric oxide (NO) concentration in the exhaled breath is suggested as a biomarker of inflammation [1]. The technique of measuring NO in exhaled breath (FENO) is a non-invasive method for evaluating chronic airway inflammation in asthma or COPD [2-10]. Currently, the presence of portable devices for measurement of FENO (fractioned exhaled NO) may help clinicians to perform this technique in daily work. Portable devices may be used to manage allergic rhinitis, asthma and other respiratory disorders such as bronchial hyperresponsiveness (BHR), chronic bronchitis, or chronic cough. The level of FENO directly reflects the inflammatory process that occurs in the airways [11].
Previous studies have shown that there is a strong correlation between FENO and increased concentrations of inflammatory biomarkers such as blood eosinophil counts, blood soluble interleukin concentrations or eosinophil level in induced sputum [3,5,11,12]. FENO, however, has a good specificity and sensitivity than other methods such lung function testing, exercise induced bronchospasm or dry air breathing for the diagnosis of asthmatic patients.
Measurement of NO in exhaled breath may help to diagnose asthma in the latent period, especially when asthmatic patients have nocturnal chronic cough as a main symptom of asthma. Moreover, the correlation between the level of FENO and BHR, a pathological feature associated with chronic inflammation in asthma [13], has also been demonstrated. This phenomenon is also implicated in the pathogenesis of asthma and chronic cough related to asthma; it is also known as a risk factor for the development of respiratory symptoms as well as a predictor of respiratory functional decline [2,14].
BHR is currently diagnosed by methacholine challenge testing. This method [14] is the most common technique for assessing an increased hyperresponsiveness. This method uses methacholine, a product that directly affects smooth muscle cells and causes bronchial smooth muscle contraction. The evaluation of MCT result is based on the methacholine concentration that decreases FEV1 (forced expiratory volume in first second) by more than 20% in adult. Methacholine challenge testing is also very useful for the diagnosis of atypical asthma [15], especially for subjects suspected asthma with chronic cough [16]. The presence of BHR associated with increased FENO may be a good evidence for treatment with anti inflammatory drugs such as leukotriene receptor antagonists or inhaled corticosteroids.
This study aimed to evaluate the level of FENO and BHR in subjects with chronic cough, the correlation between the level of FENO and concentration of PD20–methacholine, and the cut-off of FENO for diagnosis of BHR (+).
METHOD
Study subjects
Subjects with chronic cough were included in this study after signing an Institutional Review Board (IRB) approved consent and meeting the inclusion criteria at Clinical Research of LD Medical College.
Inclusion criteria: subjects with chronic cough it lasted for more than 2 weeks; no medical history with chronic bronchitis or diagnosed asthma; chronic cough was not related to ENT (ear-nose-throat) problem or GERD (gastroesophageal reflux disease); normal chest X-ray.
Exclusion criteria: active smokers; recent respiratory infection; chronic bronchitis; diagnosed asthma; iatrogenic cough; GERD; allergic rhinitis; contraindications for methacholine testing (MCT); could not do FENO measurement and spirometry.
Laboratory techniques
Measurement of exhaled NO (FENO)
FENO measurement is performed by portable device using chemiluminescence analyzer labeled NO Breath® (Bedfont; UK). To ensure compliance, the study subjects needed to exhale through the mouthpiece with a flow rate at 50 mL/s; the technician connected the mouthpiece to the NObreath® flo and perform a test blow; the ball in the flow indicator needs to be within the white band throughout the duration of the test; the monitor is easy to operate with its icon-based touchscreen display. Briefly, to power on the NObreath®, the operator attached the mouthpiece and selected the adult setting to initiate the test; keeped the ball inbetween the white areas of the visual aid to ensure correct flow into the NObreath Flo with deep breath in; when prompted, take a deep breath in and exhale until the purple status bar is filled and the NObreath® beeps. Results displayed on the screen on the NObreath FeNO monitor. The reading displayed as a single value in parts per billion on the screen in accordance to the guidance [17].
Measurement of spirometry
Spirometry was done by Blue Spiro or Body Box 500 (Medisoft, Sorinnes, Belgium). Each study subjects performed three flow curves and the best value recorded, in accordance with the ATS/ERS specification [17]. The data of spirometry was presented as a percentage of the theoretical normal value as recommended.
Measurment of BHR
BHR was measured by bronchocontraction challenge with methacholine in the form of a 5% concentration solution. Prior to each test, a solution of 2.5% methacholine was prepared by diluting the stock solution in 2 ml of saline solution (NaCl 0.9%). Study subjects in sitting posture, using nose clip and oral inhalation from an aerosol spray device automatically triggered by computer software. After the first measurement with methacholine-free diluted saline, the subject inhaled methacholine through several steps at twice the dose starting from 100 μg to a maximum cumulative dose of 3,100 μg. FEV1 was measured at 30 seconds after each step, and the lowest value was recorded. The test stops when >20% decrease in FEV1 occurs, or after reaching the maximum dose. The dose of methacholine accumulated causing 20% reduction in FEV1 value (PD20-methacholine) was calculated from linear equation of response curve / dose. BHR was positive (+) when there was a decrease in FEV1> 20% at methocholine dose ≤3,100 μg. All the study subjects who underwent methacholine challenge testing received 400 µg of salbutamol after the test to assure FEV1 post-test >80% before leaving the Research Center.
Statistical analysis
Statistical analysis was performed by using SPSS software (version 20.0, Chicago IL; USA). The comparison between the two groups was done by the Student test. Pearson coefficients were used to evaluate the correlation between parameters and statistical significance as determined by a P value <0.005. The ROC curve is used to examine the FENO predictive value for BHR.
RESULTS
Clinical and functional characteristics of study subjects
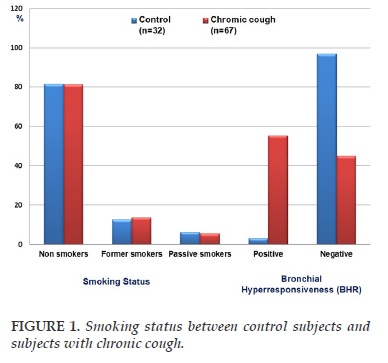
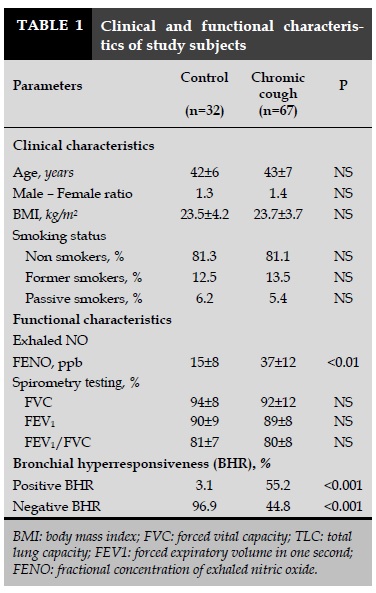
There were 32 healthy control subjects and 67 subjects with chronic cough were included in this study. Subjects were classified into two groups: group 1 consisting of 32 control subjects and group 2 with 67 subjects with chronic cough. The clinical characteristics of the study are summarized in Table 1. The mean age and male - female ratio were not different between two groups (43±7 vs 42±6 years; 1.4 vs 1.3; Table 1). The body mass index (BMI) scores did not differ between subjects with chronic cough vs controls (23.7±3.7 vs 23.5±4.2 kg/m2; Table 1). The smoking status was not significant difference between two groups (Figure 1). The level of FENO in subjects with chronic cough was significant higher than that in control subjects (37±12 vs 15±8 ppb; P <0.01; Table 1). The spirometry data was in the limit of normal in two groups. There was no obstructive ventilatory disorder (Table 1). The FEV1 in subjects with chronic cough was not significant difference in compare to control subjects (89±8 vs 90±9%; Table 1). The percentage of subjects had the positive and negative BHR in subjects with chronic cough was significantly different in compare to control subjects (55.2 and 44.8 vs 3.1 and 96.9%; P<0.001 and P<0.001; respectively).
Clinical and functional characteristics of subjects with chronic cough classified by BHR (-) or (+)
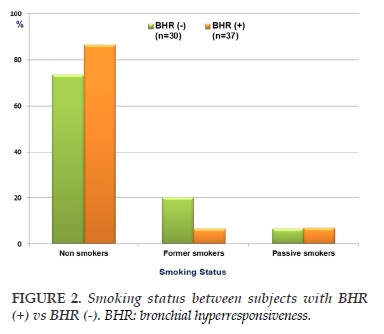
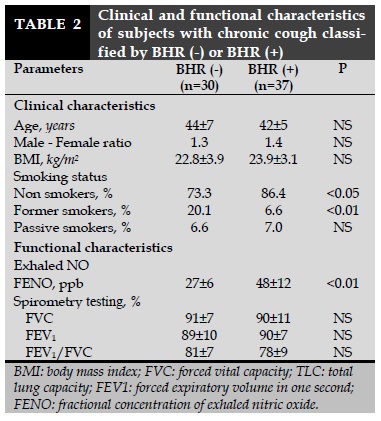
The clinical and functional characteristics of subjects with chronic cough classified by BHR positive (+) or negative (-) are presented in Table 2. There were no significant differences between subjects with BHR (-) and BHR (+) for age, male - female ratio, and BMI (Table 2). The percentage of subjects with BHR (+) and non smokers was higher than that in subjects with BHR (-) (86.4 vs 73.3%; P<0.05; Table 2) while the percentage of subjects with BHR (+) and former smokers was lower than subjects with BHR (-) (6.6 vs 20.1%; P<0.01; Table 2). The spirometric parameters were not significant differences between subjects with BHR (-) vs BHR (+). However, the level of FENO in subjects with BHR (+) was significantly higher than subjects with BHR (-) (48±12 vs 27±6 ppb; P<0.01; Table 2).
Correlation between BHR and FENO and the cut-off of FENO for diagnosis of BHR (+)
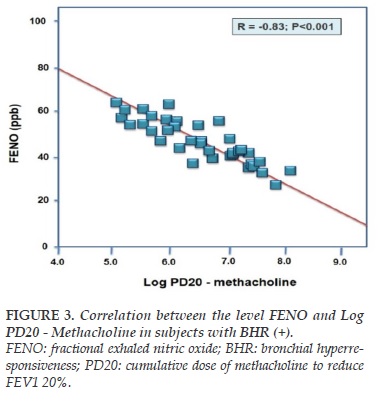
The correlation between BHR and FENO was measured by linear regression equation between the level of FENO and Log PD20-methacholine as shown in Figure 1 for subjects with BHR (+). In these subjects, FENO was tightly correlated with log PD20 (R = -0.83; P<0.001). By multivariate regression analysis, the results showed that the correlation between FENO and Log PD20 was not influenced by age, sex, BMI, and FEV1.
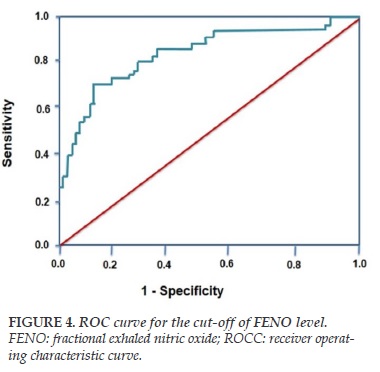
ROC curve for BHR (+) is shown in Figure 4. The cut-off of FENO level for diagnosis of positive bronchial responsiveness (BHR +) is >36 ppb with the highest sensitivity and specificity (>80% and >75%; respectively; Figure 4).
DISCUSSION
The results of the study showed that: 1) Subjects with chronic cough had increased FENO level than control subjects; 2) Subjects with chronic cough had higher percentage of bronchial hyperresponsiveness (BHR) measured by methacholine testing; 3) The level of FENO in subjects with BHR (+) was significantly higher than subjects with BHR (-); 4) There was a significant correlation between FENO level and LogPD20 – methacholine in subjects with chronic cough who had BHR (+); 5) The cut-off of FENO for diagnosis of BHR (+) with highest sensitivity and specificity was > 36 ppb. Chronic cough is a very common symptom in respiratory diseases and may be cause of different etiologies. The most common causes of chronic cough without infectious symptoms are chronic bronchitis, COPD, asthma or gastroesophageal reflux disorder (GERD). Previous studies showed that bronchial hyperresponsiveness is also frequent in subjects with chronic cough [16]. In subjects with allergy status, chronic cough may be a symptom of asthma, particularly nocturnal chronic cough [19]. In the present study, subjects with chronic cough had high level of FENO than control subjects (Table 1). The results of our previous study and the present study showed that the level of control subjects was less than 25 ppb as recommended by international guidelines. The high level of FENO in subjects with chronic cough and without the symptoms of chronic infection might due to respiratory allergy. This event may be diagnosed by methacholine challenge as recommended previously [20].
The result of present study showed that subjects with chronic cough also had a high percentage of BHR (+) than healthy subjects (control subjects) (Table 1). This result suggests that the main cause of chronic cough might be due to BHR. However, these subjects should be followed-up in to know if asthma will develop in the future. The result study also showed that subjects with chronic cough who had BHR (+) having high level of FENO that subjects with BHR (-) (Table 2).
Interestingly, there was a tight correlation between the level of FENO and Log PD20 – methacholine in subjects with chronic cough and BHR (+) (Figure 3). Moreover, the statistical analyze of ROC curve found out that the cut-off of FENO > 36 ppb had the highest sensitivity and specificity for diagnosis of BHR (+) (Figure 4). This result suggests that FENO measurement might be performed as a useful tool in diagnosis of subjects with suspected BHR, particularly for BHR in subjects with chronic cough.
CONCLUSION
The measurement of FENO with portable device is a useful tool to diagnose subjects with bronchial hyperresponsiveness, especially for subjects with chronic cough. The measurement of FENO with portable devise is affordable and feasible laboratory test for management subjects with chronic cough. However, it is necessary to study more about the mechanism for the link between increased FENO and positive bronchial hyperresponsiveness in the future.
CONFLIT OF INTEREST
Non.
REFERENCES
1. Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001 163(7):1693-722.
2. Duong-Quy S, Tran Van H, Vo Thi Kim A, Pham Huy Q, and Craig T.. Clinical and Functional Characteristics of Subjects with Asthma, COPD, and Asthma-COPD Overlap: A Multicentre Study in Vietnam. Canadian Respiratory Journal Volume 2018, Article ID 1732946, 11 pages.
3. Duong-Quy S, Vu-Minh T, Hua-Huy T, Tang-Thi-Thao T, Le-Quang K, Tran-Thanh D, Doan-Thi-Quynh N, Le-Dong NN, Craig TJ, Dinh-Xuan AT. Study of nasal exhaled nitric oxide levels in diagnosis of allergic rhinitis in subjects with and without asthma. J Asthma Allergy. 2017;22;10:75-82.
4. Nguyen Thi Bich H, Duong Thi Ly H, Vu Thi T, Phan Dinh L, Le Thi Minh H, J. Craig T, Duong-Quy S. Study of the correlations between FENO in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J Asthma Allergy. 2016 Sep 14;9:163-170.
5. K Dang Thi Mai, Y Nguyen Hoang, B Nguyen Quoc, K Le Quang, S Duong-Quy. Study of exhaled NO in subjects without apneas during sleep. J Func Vent Pulm2015 ; 6 (17), 36-42.
6. Duong-Quy S; ,Etude sur la mesure du NO exhalé dans la prise en charge des patients asthmatiques, J Func Vent Pulm2013,4: 11,23-28.
7. Duong-Quy, S; Rôle des différents paramètres du NO expiré dans la détection de l’hyperréactivité bronchique,J Func Vent Pulm2012 ; 8: 52-59.
8. Nguyen Thi Hong, L; Pham Van, T; Duong-Quy, S; Phan Quang, D; Nguyen Van, Doan; ,Etude sur le rôle du NO exhalé dans la prise en charge des patients asthmatiques,J Func Vent Pulm2012 ; 7 : 29-35.
9. Vo Pham Minh, T; Duong-Quy, S; Ta Ba, T; Nguyen Viet, N; ,Value of exhaled NO in COPD: « results and discussion »,J Func Vent Pulm2012: ,7,23-28.
10. Duong-Quy, S; Pham-Van, L; ,Valeur diagnostique du NO exhalé chez les patients asthmatiques vietnamiens,J Func Vent Pulm2012 ; 6,41-46.
11. Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001 163(7):1693-722.
12. A Jatakanon, S Lim, S A Kharitonov, K F Chung, P J Barnes. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 1998; 53: 91–95 91.
13. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000; 161: 1720–1745.
14. O’Connor G, Sparrow D, Weiss S. A prospective longitudinal study of methacholine airway responsiveness as a predictor of pulmonary-function decline: the Normative Aging Study. Am J Respir Crit Care Med 1995; 152: 87–92.
15. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161(1):309-29.
16. N. Scichilone, M. Messina, S. Battaglia, F. Catalano, V. Bellia. Airway hyperresponsiveness in the elderly: prevalence and clinical implications. Eur Respir J 2005; 25: 364–375.
17. American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 15;171(8):912-30.
18. Andrew D. Smith, Jan O. Cowan, Sue Filsell, Chris MacLachlan, Gabrielle Monti-Sheehan, Pamela Jackson and D. Robin Taylor. Diagnosing Asthma: Comparisons between Exhaled Nitric Oxide Measurements and Conventional Tests. Am J Respir Crit Care Med Vol 169. pp 473-478, 2004.;
19. Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118(3):551-9.
20. Berkman N, Avital A, Breuer R, Bardach E, Springer C, Godfrey S. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax. 2005;60(5):383-8.
FIGURES - TABLES
REFERENCES
1. Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001 163(7):1693-722.
2. Duong-Quy S, Tran Van H, Vo Thi Kim A, Pham Huy Q, and Craig T.. Clinical and Functional Characteristics of Subjects with Asthma, COPD, and Asthma-COPD Overlap: A Multicentre Study in Vietnam. Canadian Respiratory Journal Volume 2018, Article ID 1732946, 11 pages.
3. Duong-Quy S, Vu-Minh T, Hua-Huy T, Tang-Thi-Thao T, Le-Quang K, Tran-Thanh D, Doan-Thi-Quynh N, Le-Dong NN, Craig TJ, Dinh-Xuan AT. Study of nasal exhaled nitric oxide levels in diagnosis of allergic rhinitis in subjects with and without asthma. J Asthma Allergy. 2017;22;10:75-82.
4. Nguyen Thi Bich H, Duong Thi Ly H, Vu Thi T, Phan Dinh L, Le Thi Minh H, J. Craig T, Duong-Quy S. Study of the correlations between FENO in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J Asthma Allergy. 2016 Sep 14;9:163-170.
5. K Dang Thi Mai, Y Nguyen Hoang, B Nguyen Quoc, K Le Quang, S Duong-Quy. Study of exhaled NO in subjects without apneas during sleep. J Func Vent Pulm2015 ; 6 (17), 36-42.
6. Duong-Quy S; ,Etude sur la mesure du NO exhalé dans la prise en charge des patients asthmatiques, J Func Vent Pulm2013,4: 11,23-28.
7. Duong-Quy, S; Rôle des différents paramètres du NO expiré dans la détection de l’hyperréactivité bronchique,J Func Vent Pulm2012 ; 8: 52-59.
8. Nguyen Thi Hong, L; Pham Van, T; Duong-Quy, S; Phan Quang, D; Nguyen Van, Doan; ,Etude sur le rôle du NO exhalé dans la prise en charge des patients asthmatiques,J Func Vent Pulm2012 ; 7 : 29-35.
9. Vo Pham Minh, T; Duong-Quy, S; Ta Ba, T; Nguyen Viet, N; ,Value of exhaled NO in COPD: « results and discussion »,J Func Vent Pulm2012: ,7,23-28.
10. Duong-Quy, S; Pham-Van, L; ,Valeur diagnostique du NO exhalé chez les patients asthmatiques vietnamiens,J Func Vent Pulm2012 ; 6,41-46.
11. Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001 163(7):1693-722.
12. A Jatakanon, S Lim, S A Kharitonov, K F Chung, P J Barnes. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 1998; 53: 91–95 91.
13. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000; 161: 1720–1745.
14. O’Connor G, Sparrow D, Weiss S. A prospective longitudinal study of methacholine airway responsiveness as a predictor of pulmonary-function decline: the Normative Aging Study. Am J Respir Crit Care Med 1995; 152: 87–92.
15. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161(1):309-29.
16. N. Scichilone, M. Messina, S. Battaglia, F. Catalano, V. Bellia. Airway hyperresponsiveness in the elderly: prevalence and clinical implications. Eur Respir J 2005; 25: 364–375.
17. American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 15;171(8):912-30.
18. Andrew D. Smith, Jan O. Cowan, Sue Filsell, Chris MacLachlan, Gabrielle Monti-Sheehan, Pamela Jackson and D. Robin Taylor. Diagnosing Asthma: Comparisons between Exhaled Nitric Oxide Measurements and Conventional Tests. Am J Respir Crit Care Med Vol 169. pp 473-478, 2004.;
19. Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118(3):551-9.
20. Berkman N, Avital A, Breuer R, Bardach E, Springer C, Godfrey S. Exhaled nitric oxide in the diagnosis of asthma: comparison with bronchial provocation tests. Thorax. 2005;60(5):383-8.
ARTICLE INFO
DOI: 10.12699/jfvpulm.9.27.2018.11
Conflict of Interest
Non
Date of manuscript receiving
09/03/2018
Date of publication after correction
31/08/2018
Article citation
Pham-Huy Q, Tran-Ngoc T, Nguyen-Van T, Nguyen-Quoc B, Le-Quang K, Tran-Thanh D, Doan-Thi-Quynh N, Craig T, Duong-Quy S. Study of correlation between exhaled NO measured by portable device and bronchial hyperresponsiveness in subjects with chronic cough. J Func Vent Pulm 2018;27(9):11-16.