
 English
English
 French
French
Study of skin prick test, total IgE, blood eosinophil and exhaled nitric oxide (FENO) for classifying allergic or non-allergic asthma and for asthma management
Étude sur le test cutané, IgE totale, éosinophile sanguin et la fraction du monoxide d’azote dans l’air expiré (FENO) pour classifier l’asthme allergique ou non-allergique et pour le traitement
Luu Minh Chau1, Q. Pham-Huy2, S. Duong Quy3,4
1 ; Vietnam University of Traditional Medicine. Hanoi, Vietnam.
2; Hai Phong University of Medicine and Pharmacy, Vietnam
3; Clinical Research Center. Lam Dong Medical College. Vietnam
4: Penn State Medical College. Hershey Medical Center. USA
Corresponding author
Dr. DUONG-QUY Sy
Lam Dong Medical College. Penn State University of Medicine
Email: sduongquy.jfvp@gmail.com
ABSTRACT
Background. Asthma is a common respiratory disorder, characterized by local infiltration and activation of a variety of inflammatory mediators and cells. In allergic asthma, increased blood or tissue eosinophilia, total concentration or specific IgE, fraction exhaled nitric oxide are frequently reported.
Method. It was a prospective study on 67 asthmatic patients. Eosinophil count, IgE concentrations, lung function, and measurements of nitric oxide in exhaled breath (FENO) were done. The patient had positive skin prick test were classified as allergic asthma patients and patients with negative skin prick test were considered as non-allergic asthmatic patients. Results. More than 70% of patients had a history of allergy, in which allergic rhinitis is the most common comorbidity. Positive skin test of Df, Dp and Blomia house dust mites were higher. Total IgE and blood eosinophil level were high than normal limit. FENO concentrations were higher in allergic asthmatic patients. All asthmatic patients had a good response to ICS with moderate to high doses. The asthma control was achieved after 6 months of standard treatment.
Conclusion. Asthmatic patients have mainly allergic characteristics with increased inflammatory biomarkers, especially FENO level. Therefore, the use of FENO is useful for management of asthma.
KEYWORDS: Asthma, skin prick test, IgE, eosinophila, nitric oxide, FENO.
RÉSUMÉ
Contexte. L'asthme est une maladie respiratoire courante caractérisée par une infiltration locale et l'activation de divers médiateurs et cellules inflammatoires. Chez les asthmatiques, une augmentation de l'éosinophilie sanguine ou tissulaire, une concentration totale ou des IgE spécifiques, de monoxide d’azote (NO) dans l’air expiré sont souvent rapportés.
Méthode. Il s'agissait d'une étude prospective sur 67 patients asthmatiques. La numération des éosinophiles, les concentrations d’ IgE, la fonction respiratoire et la mesure du NO dans l'air exhalée (FENO) ont été effectuées. Les patients avec un test cutané positif ont été classés comme patients asthmatiques allergiques et les patients avec un test cutané négatif ont été considérés comme des patients asthmatiques non allergiques.
Résultats. Plus de 70% des patients avaient des antécédents d'allergie, dans laquelle la rhinite allergique est la comorbidité la plus courante. Les tests cutanés positifs pour les acariens de la poussière de maison Df, Dp et Blomia étaient plus fréquents. Les niveaux d'IgE totales et d'éosinophiles étaient plus élevés que la normale. Les concentrations de FENO étaient plus élevées chez les patients asthmatiques allergiques. Tous les patients asthmatiques ont bien répondu aux CSI avec des doses modérées à élevées. Le contrôle de l'asthme a été atteint après 6 mois de traitement standard.
Conclusion. Les patients asthmatiques ont principalement des caractéristiques allergiques avec une augmentation des biomarqueurs inflammatoires, en particulier le niveau de FENO. L'utilisation de FENO est utile pour la gestion de l'asthme.
MOTS CLÉS: Asthme, test cutané, IgE, hypereosinophil, monoxide d’azote, FENO.
INTRODUCTION
Asthma is a common respiratory disorder, characterized by local infiltration and activation of a variety of inflammatory mediators and cells. The finding that eosinophils are harmful to human lung tissue and that their presence in the bronchial mucosa may correlate with morphological damage to the bronchial [1]. Non-invasive markers (biomarkers) to assess the presence and the intensity of airway inflammation in adult and children have been recommended. Measurements of several blood markers of inflammation have been reported in the monitoring of asthma [2,3] as well as fraction exhaled nitric oxide (FENO) has been proposed as to assess airway inflammation in asthmatic patients [4-8].
Measuring the level of nitric oxide (NO) in the diagnosis and treatment of asthma has been studied for several years [4-6] and today has become a popular tool in many countries due to its use. Handheld NO-devices are very convenient and useful. The application of the FENO method in the asthma management guidelines has created a new step in the diagnosis and treatment of asthma and has been included in international recommendations [6,9]. Increased FENO directly reflects inflammation in the airways in asthmatic patients and has a strong correlation between FENO and treatment response [10-15].
However, FENO is not only used as a marker of inflammation in asthma as it also correlates with the incidence of asthma symptoms, as well as the level of asthma control. Excessive fluctuation of FENO can predict asthma attacks. In addition, FENO measurements are also used for the purpose of evaluating treatment response and optimizing treatment with inhaled corticosteroid. Due to the close correlation between FENO and response to inhaled corticosteroid therapy. Initial studies have shown that NO decreases with inhaled and systemic corticosteroid therapy, which is rapid and inversely proportional to the dose administered [14-17]. Increasing or decreasing FENO values between the two measurements is a sign of decay or improvement in the level of asthma control.
In Vietnam, the study on FENO measurement in treatment guidelines in asthma patients is still very little. Therefore, the study of FENO treatment for asthma patients is necessary.
OBJECTIVES
This study was conducted to evaluate the allergy status of asthmatic patients and the effect of using FENO in diagnosis and treatment of allergic patients.
METHOD
Study subjects
Patients >18-year old who were diagnosed with asthma at the Clinical Research Center of Bio-Medical Center of Lam Dong Medical College from January 2016 to January 2017.
Selection criteria for study subjects
Inclusion criteria
Subjects were more than 18 years.
Have not been in asthma control (never treated or non-diagnosed patients) or quit at least three month of daily treatment.
Correctly realized all clinical and functional techniques as recommended.
Exclusion criteria
Patients with asthma accompanied by other severe diseases.
Patients with severe asthma attacks.
Patients being treated with systemic corticosteroids (oral, injectable).
Criteria for diagnosis of asthma
Following 2016 Global Initiative for Asthma (GINA) guidelines for adult to diagnose asthma, asthma severity, and asthma exacerbation.
Methods
Study design
It was a prospective study with the intervention for asthma treatment with inhaled corticosteroid according to GINA 2016 guidelines. This study was approved to the Ethic Review Board (ERB) of Lam Dong Medical College.
There was sixty-seven patients were enrolled and performed skin prick test (SPT), lung function, and exhaled NO and total IgE measurements.
If the patient has positive skin prick test, we class allergic asthma patient and patient with negative skin prick test was non-allergic asthma patient.
Patient classification
Included patients were selected for the study group.
Patient classification was categorized at intermittent, mild, moderate, and severe asthma; and asthma control of GINA 2016 as following uncontrolled, partially controlled, and controlled.
Data collection
All the patients were asked for medical information and clinical examination to determine asthma status and levels.
Family and patients’ history of allergies such as allergic rhinitis, allergic conjunctivitis, eczema, urticaria, drug allergy, food allergy, and allergic asthma.
Laboratory tests
Lung function test (LFT)
Performed by phlethysmography or spirometry instruments. LFT was done by using whole-body phlethysmography with Body Box 500 (Medisoft, Sorinnes, Belgium).
The Body Box had been calibrated every day by using a standard three liter pump. Al the data such as FVC, FEV1, FEV1/FVC, PEF, FEF25-75 were recorded for analyses. Each study subject beneficed three best flow - volume curves for FEV1 and all values were selected in accordance with the ATS (American Thoracic Society)/ERS (European Respiratory Society) recommendations (11). The data was presented as percentage of theoretical normal values.
Blood count
The number of total IgE, and eosinophilia was done by automatical machine.
Total amount of IgE in the blood was measured by luminescence chemistry techniques.
Skin prick test (SPT)
Negative control is a saline solution 0.9%; positive control is histamine 1mg/ml. Six respiratory allergens are made: Dermatophagoides Pteronyssius (Dp), Dermatophagoides Farinae (Df), Blomia tropicalis (Blo), hair and epidermis of dogs, cats, cockroaches (Stallergen; UK). SPT is positive when redpapular area ≥3x3 mm.
Measurement of exhaled NO
Exhaled NO was measured at multiple flow rates (50 mL/s, 100 mL/s, 150 mL/s, and 350 mL/s) before methacholine challenge by using an electrochemical based analyzer FeNO+ (Medisoft, Sorinnes, Belgium).
Technical measurement of exhaled NO was conducted according to manufacturer’s instructions, as recommended by the ATS/ERS guideline [6]. The maximal bronchial production rate of NO (J’awNO) and alveolar concentration of NO (CANO) were automatically determined using the two-compartment model and had been reported via Expair’s software (Medisoft) as described previously [12,13].
Statistical analyses
Statistical analysis was performed by using SPSS software (version 22.0; Chicago IL; USA). Values were expressed as mean ± standard deviation for quantitative variables and percentage for qualitative variables. The comparison between groups was done by Student’s t-test. The Pearson coefficient was used to evaluate the correlation between parameters and statistical significance as determined by P value < 0.05.
RESULTS
Clinical and functional des study subjects
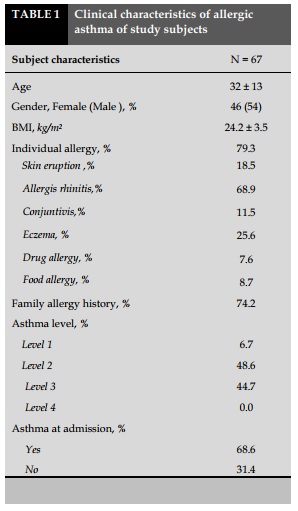
Clinical characteristics of asthmatic patient are presented in Table 1.
Average age of study group was 32±13 years old with the average BMI of 24.2±3.5.
The ratio of male/female was 0.8/1.2; most of patients had allergic history with the percentage of 79.3%, in which patients with allergic rhinitis were the most common with 68.9%.
There was 74.2% of asthmatic patients had medical history of allergy. The majority of patients had asthma with level 2 and 3 of severity with a rate 48.6% and 44.7%, respectively.
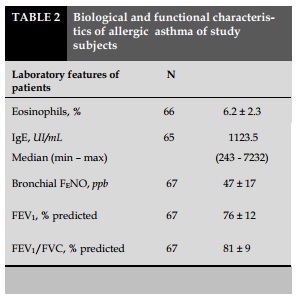
The lung function of asthmatic patients decreased slightly, with FEV1 of 76±12% of the predicted theory (Table 2). The concentration of exhaled nitric oxide (NO) levels were increased by 47±17 ppb. FENO was increased highly. IgE levels and eosinophils were significantly higher than normal.
Most patients were allergic to respiratory allergens, most commonly allergic to house dust mites.
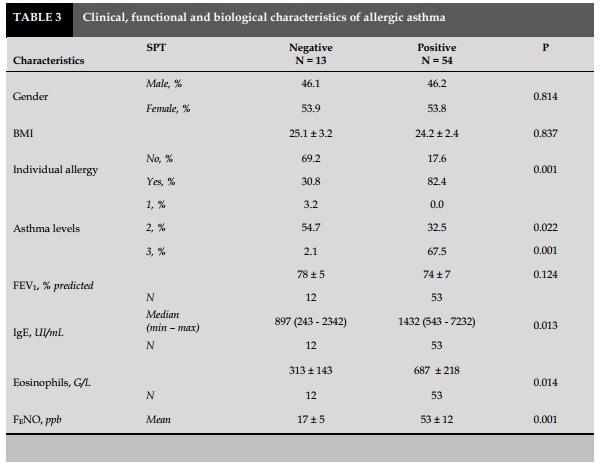
There was no significant difference about gender (male/female), BMI, or lung function in patients with positive and negative skin prick test (SPT) with P> 0.05 (Table 3).
Patients with positive respiratory allergen as house dust mites, dog hair, cat hair or crockroach had high percentage vs negative patient with significant difference with P<0.05 (Table 3).
Compare with non-allergic asthma patients evaluated by SPT, allergic asthma patients had significantly higher degree of blood eosinophilia 687±218 G/L vs 313±143 G/L; total concentration of IgE 1432 UI/mL vs 897.0 UI/mL with P=0.014 and P=0.013, respectively (Table 3).
Exhaled NO measurements showed that patient with allergic asthma had FENO level was higher than that in non-allergic but no significant difference (53±12 vs 17±5; P=0.001; Table 3).
There was no significant difference in FEV1 value between non-allergic and allergic patients (Table 3).
Response to treatment of asthmatic patients
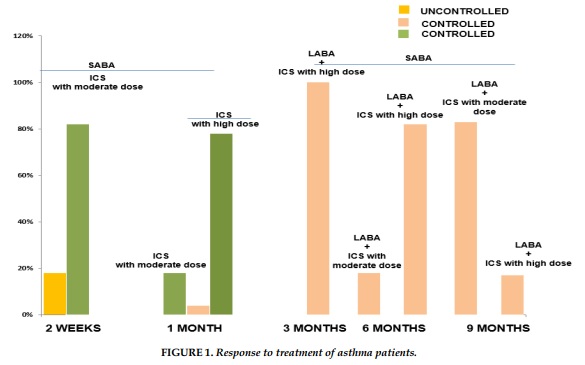
The results of the study showed that the complete control of asthmatic patients was achieved after 3 months with high doses of inhaled corticosteroid (ICS) for all study subjects. The proportion of patients with complete control of asthma after 6 months with long acting bronchodilator (LABA) and high dose ICS in asthmatic patients who were treated following FENO level was higher than patients treated by GINA recommendations plus FENO and GINA alone. The result showed that the daily dose of ICS in asthmatic patients treated following FENO level was significantly higher than GINA at 9 months (Figure 1).
DISCUSSION
The result of present study showed that asthmatic patients had a history of allergy such as eczema, allergic rhinitis, allergic conjunctivitis, recurrent urticaria, drug allergies, and food allergies. Previous studies, which have shown that almost asthmatic patients were allergic asthma [18-20]. In these cases, allergic rhinitis is the most common co-morbidity of asthmatic patients.
The majority of patients in the study have mild to moderate persistent asthma with mild airway obstruction. In the present study, the use of fractional exhaled nitric oxide (FENO) is a non-invasive, safe, and reliable technique for assessing airway inflammation [21-24]. Previous studies have shown a link between FENO and eosinophils in sputum and in peripheral blood.
FENO reflects eosinophilic inflammation in bronchial asthma and is used as a biomarker of inflammation in asthma. In the present study, FENO concentrations were higher than normal (normal FENO <25 ppb in healthy adult). As recommended by ATS/ERS, FENO increasing is a relevant marker of increased eosinophilia inflammation and response to ICS [10]. In the present study, the total IgE concentration in the study was highly increased than normal limit. In addition, the peripheral blood eosinophilia was also increased.
The results of the study showed that the complete control of asthmatic patients was done after treatment with high doses of inhaled corticosteroid (ICS) for all asthmatic patients. The proportion of patients with complete control of asthma after long acting bronchodilator (LABA) and high dose ICS in asthmatic patients treated following FENO level was higher than patients treated by GINA + FENO and GINA alone. Moreover, the daily dose of ICS in asthmatic patients titrated by FENO level was significantly higher than that followed by GINA.
CONCLUSION
Asthmatic patients have mainly allergic characteristics with increased inflammatory biomarkers, especially FENO level. The use of FENO in combination with international recommendations in monitoring therapy for asthma control seems to be more advantageous than using because of the reduction of daily ICS dose.
CONFLIT OF INTERESTS
Non.
REFERENCES
1. Ohashi Y, Motojima S, Fukuda T et al. Airway hyperresponsiveness, increased intracellular spaces of bronchial epithelium, and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am Rev Respir Dis, 1992; 145 (6), 1469-1476.
2. Nelson BV, Sears S, Woods J et al. Expired nitric oxide as a marker for childhood asthma. J Pediatr, 1997; 130 (3), 423-427.
3. Global Initiative for Asthma (GINA); 2015. Available from: http://ginasthma.org/archived-reports/. Accessed April 13th, 2016.
4. Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 1993; 6: 1368-70.
5. American Thoracic Society. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children. Am J Respir Crit Care Med 1999; 160:2104–2117.
6. American Thoracic Society/European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 2005;171:912–30.
7. Battaglia S, den Hertog H, Timmers MC, Lazeroms SP, Vignola AM, Rabe KF, Bellia V, Hiemstra PS, Sterk PJ.Small airways function and molecular markers in exhaled air in mild asthma. Thorax. 2005;60:639-44.
8. Dupont, L.J., Demedts, M.G., Verleden, G.M. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2003; 123, 751–756.
9. Dweik RA, Boggs PB, Erzurum SC et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med, 2011;184 (5), 602-615.
10. Duong-Quy, S; Rôle des différents paramètres du NO expiré dans la détection de l’hyperréactivité bronchique,J Func Vent Pulm 2012 ; 8: 52-59.
11. Nguyen Thi Hong, L; Pham Van, T. Duong-Quy, S; Phan Quang, D; Nguyen Van, Doan; ,Etude sur le rôle du NO exhalé dans la prise en charge des patients asthmatiques,J Func Vent Pulm2012 ; 7 : 29-35.
12. Vo Pham Minh, T; Duong-Quy, S. Ta Ba, T; Nguyen Viet, N; ,Value of exhaled NO in COPD: « results and discussion »,J Func Vent Pulm 2012;7,23-28.
13. Jatakanon, A., Kjaritonov, S.A., Lim, S., Barnes, P.J. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax 1999; 54, 108–114.
14. Jones, S.L., Herbison, P., Cowan, J.O., Flannery, E.M., Hancox, R.J., McLachlan, C.R., Taylor, D.R. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose–response relationship. Eur. Respir. J. 2002; 20, 601–608.
15. Kharitonov, S.A., Yates, D.H., Barnes, P.J. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am. J. Respir. Crit. Care Med. 1996; 153, 454–457.
16. Sippel JM, Holden WE, Tilles SA, O'Hollaren M, Cook J, Thukkani N, Priest J, Nelson B, Osborne ML. Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol. 2000 Oct;106(4):645-50.
17. Smith AD, Cowan JO, Brassett KP, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005; 352:2163–2173.
18. Duong-Quy S, Vu-Minh T, Hua-Huy T, Tang-Thi-Thao T, Le-Quang K, Tran-Thanh D, Doan-Thi-Quynh N, LeDong NN, Craig TJ, Dinh-Xuan AT. Study of nasal exhaled nitric oxide levels in diagnosis of allergic rhinitis in subjects with and without asthma. J Asthma Allergy. 2017;22;10:75-82.
19. Huong Duong-Thi-Ly, Ha Nguyen-Thi-Thu, Long Nguyen-Hoang, Hanh Nguyen-Thi-Bich, Timothy J. Craig, and Duong-Quy S. Effects of genetic factors to inhaled corticosteroid response in children with asthma: a literature review. J Int Med Res 2017; 1–13.
20. Nguyen Thi Bich H, Duong Thi Ly H, Vu Thi T, Phan Dinh L, Le Thi Minh H, J. Craig T, Duong-Quy S. Study of the correlations between FENO in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J Asthma Allergy. 2016 Sep 14;9:163-170.
21. Duong-Quy S. Etude sur la mesure du NO exhalé dans la prise en charge des patients asthmatiques, J Func Vent Pulm2013,4: 11,23-28.
22. Park YA, Park HB, Kim YH, et al. Airway hyperresponsiveness to mannitol and methacholine and exhaled nitric oxide in children with asthma. J Asthma. 2017 Jan 5:1-8. doi: 10.1080/02770903.2016.1255751. [Epub ahead of print].
23. Lee JW, Shim JY, Kwon JW, et al. Exhaled nitric oxide as a better diagnostic indicator for evaluating wheeze and airway hyperresponsiveness in preschool children. J Asthma. 2015;52(10):1054-9.
24. Duong-Quy S, Hua-Huy T, Doan-Quynh N, et al. A study of exhaled NO (FENO) measurement used to determine asthma control, dose of inhaled corticosteroid and cost in a developing country. Eur Respir J. 2015;46(Suppl 59):5013.
FIGURE - TABLES
REFERENCES
1. Ohashi Y, Motojima S, Fukuda T et al. Airway hyperresponsiveness, increased intracellular spaces of bronchial epithelium, and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am Rev Respir Dis, 1992; 145 (6), 1469-1476.
2. Nelson BV, Sears S, Woods J et al. Expired nitric oxide as a marker for childhood asthma. J Pediatr, 1997; 130 (3), 423-427.
3. Global Initiative for Asthma (GINA); 2015. Available from: http://ginasthma.org/archived-reports/. Accessed April 13th, 2016.
4. Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 1993; 6: 1368-70.
5. American Thoracic Society. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children. Am J Respir Crit Care Med 1999; 160:2104–2117.
6. American Thoracic Society/European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 2005;171:912–30.
7. Battaglia S, den Hertog H, Timmers MC, Lazeroms SP, Vignola AM, Rabe KF, Bellia V, Hiemstra PS, Sterk PJ.Small airways function and molecular markers in exhaled air in mild asthma. Thorax. 2005;60:639-44.
8. Dupont, L.J., Demedts, M.G., Verleden, G.M. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2003; 123, 751–756.
9. Dweik RA, Boggs PB, Erzurum SC et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med, 2011;184 (5), 602-615.
10. Duong-Quy, S; Rôle des différents paramètres du NO expiré dans la détection de l’hyperréactivité bronchique,J Func Vent Pulm 2012 ; 8: 52-59.
11. Nguyen Thi Hong, L; Pham Van, T. Duong-Quy, S; Phan Quang, D; Nguyen Van, Doan; ,Etude sur le rôle du NO exhalé dans la prise en charge des patients asthmatiques,J Func Vent Pulm2012 ; 7 : 29-35.
12. Vo Pham Minh, T; Duong-Quy, S. Ta Ba, T; Nguyen Viet, N; ,Value of exhaled NO in COPD: « results and discussion »,J Func Vent Pulm 2012;7,23-28.
13. Jatakanon, A., Kjaritonov, S.A., Lim, S., Barnes, P.J. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax 1999; 54, 108–114.
14. Jones, S.L., Herbison, P., Cowan, J.O., Flannery, E.M., Hancox, R.J., McLachlan, C.R., Taylor, D.R. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose–response relationship. Eur. Respir. J. 2002; 20, 601–608.
15. Kharitonov, S.A., Yates, D.H., Barnes, P.J. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am. J. Respir. Crit. Care Med. 1996; 153, 454–457.
16. Sippel JM, Holden WE, Tilles SA, O'Hollaren M, Cook J, Thukkani N, Priest J, Nelson B, Osborne ML. Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol. 2000 Oct;106(4):645-50.
17. Smith AD, Cowan JO, Brassett KP, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005; 352:2163–2173.
18. Duong-Quy S, Vu-Minh T, Hua-Huy T, Tang-Thi-Thao T, Le-Quang K, Tran-Thanh D, Doan-Thi-Quynh N, LeDong NN, Craig TJ, Dinh-Xuan AT. Study of nasal exhaled nitric oxide levels in diagnosis of allergic rhinitis in subjects with and without asthma. J Asthma Allergy. 2017;22;10:75-82.
19. Huong Duong-Thi-Ly, Ha Nguyen-Thi-Thu, Long Nguyen-Hoang, Hanh Nguyen-Thi-Bich, Timothy J. Craig, and Duong-Quy S. Effects of genetic factors to inhaled corticosteroid response in children with asthma: a literature review. J Int Med Res 2017; 1–13.
20. Nguyen Thi Bich H, Duong Thi Ly H, Vu Thi T, Phan Dinh L, Le Thi Minh H, J. Craig T, Duong-Quy S. Study of the correlations between FENO in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J Asthma Allergy. 2016 Sep 14;9:163-170.
21. Duong-Quy S. Etude sur la mesure du NO exhalé dans la prise en charge des patients asthmatiques, J Func Vent Pulm2013,4: 11,23-28.
22. Park YA, Park HB, Kim YH, et al. Airway hyperresponsiveness to mannitol and methacholine and exhaled nitric oxide in children with asthma. J Asthma. 2017 Jan 5:1-8. doi: 10.1080/02770903.2016.1255751. [Epub ahead of print].
23.Park YA, Park HB, Kim YH, et al. Airway hyperresponsiveness to mannitol and methacholine and exhaled nitric oxide in children with asthma. J Asthma. 2017 Jan 5:1-8. doi: 10.1080/02770903.2016.1255751. [Epub ahead of print].
24. Duong-Quy S, Hua-Huy T, Doan-Quynh N, et al. A study of exhaled NO (FENO) measurement used to determine asthma control, dose of inhaled corticosteroid and cost in a developing country. Eur Respir J. 2015;46(Suppl 59):5013.
ARTICLE INFO
DOI: 10.12699/jfvpulm.9.29.2018.38
Conflict of Interest
Non
Date of manuscript receiving
21/06/2018
Date of publication after correction
17/12/2018
Article citation
Minh Chau Luu, Pham-Huy Q, Duong Quy S. Study of skin prick test, total IgE, blood eosinophil and exhaled nitric oxide (FENO) for classifying allergic or non-allergic asthma and for asthma management. J Func Vent Pulm 2018;29(9):38-43.