
 English
English
 French
French
Increase of alveolar nitric oxide concentration in patients with sleep apnea and its correlation with nocturnal hypoxemia and sleep apnea severity
Augmentation de la concentration d'oxyde nitrique alvéolaire chez les patients atteints d'apnée du sommeil et corrélation avec l'hypoxémie nocturne et la sévérité de l'apnée du sommeil
Luu Minh Chau1, T. Tran-Thi-Khanh2, S. Duong Quy3,4
1 ; Vietnam University of Traditional Medicine. Hanoi, Vietnam.
2; Pham Ngoc Thach University of Medicine. Ho Chi Minh city, Vietnam
3; Clinical Research Center. Lam Dong Medical College. Vietnam
4: Penn State Medical College. Hershey Medical Center. USA
Corresponding author
Dr. LUU MINH Chau
Vietnam University of Traditional Medicine. Hanoi - Vietnam
Email: minhchauytdp@gmail.com
ABSTRACT
Background. Obstructive sleep apnea syndrome (OSAS) is characterized by periodic obstruction of pharynx, leading to respiratory airways inflammation and endothelial dysfunction. The concentration of exhaled NO has been used to evaluate and monitor both phenomena.
Method. Prospective study on 116 patients with suspected OSAS symptoms. Respiratory function, polysomnography, and exhaled NO measured had been done for each patient. Patients with apnea-hyponea index (AHI) <5/hour were included in control group.
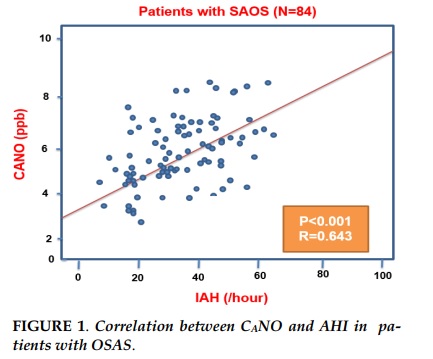
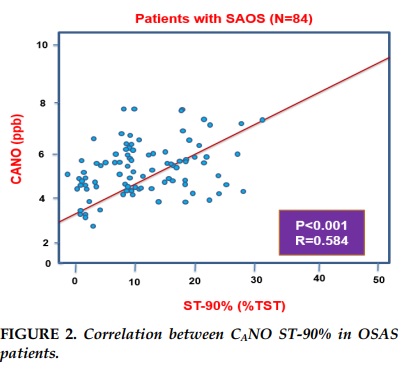
Results. Alveolar NO concentration (CANO) was significantly increased in patients with OSAS (N=84; P<0,001) in comparison with control subjects (N=32). There were no significant differences between two groups for bronchial NO concentration (J’awNO) and total NO concentration (FENO) (P>0,05). In OSAS group, there were the linear correlations between CANO and AHI (R=0.643; P<0,001), CANO and the ratio of sleep time with SpO2 <90% in total sleep time (TS-90%) (R=0.584; P<0,001).
Conclusion. The result shows that CANO is correlated with the severity of AHI and noctural hypoxemia in patients with obstructive sleep apnea syndrome.
KEYWORDS: Nitric oxide, NO, FENO, CANO, J’awNO, OSAS .
RÉSUMÉ
Introduction. Le syndrome d'apnées obstructives du sommeil (SAOS) est caractérisé par une obstruction périodique du pharynx, entraînant une inflammation des voies respiratoires et un dysfonctionnement endothélial. La concentration de NO expiré a été utilisée pour évaluer et surveiller ces deux phénomènes.
Méthode. Étude prospective sur 116 patients suspectés de symptômes du SAOS. La fonction respiratoire, la polysomnographie et le NO expire avaient été effectués pour chaque patient. Les patients ayant un indice d'apnée hyponea (IAH)<5/ heure ont été inclus dans le groupe contrôle.
Résultats. La concentration de NO alvéolaire (CANO) était significativement augmentée chez les patients atteints de SAOS (N=84; P<0,001) par rapport aux sujets témoins (N=32). Il n’existait aucune différence significative entre la concentration de NO bronchique (J’awNO) et la concentration de NO totale (FENO) bronchique (P>0,05) entre deux groupes. Dans le groupe SAOS, il existait les corrélations linéaires entre CANO et IAH (R= 0,643; P<0,001), CANO et le rapport du temps de sommeil avec SpO2 <90% du temps total de sommeil (TS-90%) (R=0,584; P<0,001).
Conclusion. Le résultat montre que CANO est en corrélation avec la gravité de l'IAH et de l'hypoxémie nocturne chez les patients atteints du syndrome d'apnées obstructives du sommeil.
MOTS CLÉS: Nitric oxide, NO, FENO, CANO, J’awNO, SAOS.
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a high risk factor for cardiovascular morbidity and mortality, caused by periodic airway obstruction resulting in increased production of reactive oxygen species (ROS) and proinflammatory cytokines. As a result, oxidative stress and inflammation can lead to endothelial dysfunction, which can lead to cardiovascular diseases [1,2]. Therefore, the assessment of inflammation in lungs may be useful in screening for OSAS as well as in predicting the severity of the disease.
Nitric oxide (NO) plays an important role as a physiological regulator of vascular force and as a preinflammatory marker in many pulmonary diseases. NO can be readily measured in the patient's exhalation and is therefore used to assess the severity of multiple pulmonary diseases: increased NO in inflammation and decreased NO in endothelial dysfunction [3]. Both can occur in OSAS patients depending on the severity and progression of the disease. FENO reflects the production of NO over the entire airway, may remain unchanged or increased in OSAS patients.
However, this parameter does not indicate the amount of NO released from the peripheral part of the lung, i.e. in the bronchi and alveoli. The twochamber model of measurement (alveolar and airway) allows the determination of maximal J'awNO and CANO by basic mathematical formula [4].
We measured NO in exhalation in patients with the suspicion of OSAS and polysomnography, to find out the correlations between NO parameters and associated parameters with the severity of OSAS. The main purpose of the study was to consider the possibility of OSAS assessment by measuring NO in exhalation breath.
METHODS
Study population
All patients from 18 years and up who came to the Sleep Lab Center of Lam Dong Medical College (LMC) - Dalat, with the suspected OSAS.
The clinical symptoms were consistent with the standards of American Association of Sleep Medicine (AASM) [5]. The study was approved by the Research Institute Board of LMC and all participating patients signed a written agreement.
Patients with severe cardiovascular disease (level 2 heart failure and up, according to NYHA) or severe pulmonary pathology (congestive or restrictive respiratory failure) were not included in the study.
Patients who smoked, had respiratory infections or were taking oral steroids within 4 weeks before the study are excluded from the study because these factors affect exhaled NO levels [4].
All patients were asked for a medical history and had general clinical examination before participating in the study. Patients who agreed to participate in the study would be scheduled for another day to measure NO and respiratory function in the afternoon, followed by performing polysomnography or respiratory polygraphy at night.
Healthy subjects with non-smokers people agreed to participate in NO measurement in exhalation were selected as the control group.
Measuring respiratory function
Respiratory function tests include the measurement of vital capacity (VC), forced expiratory volume in one second (FEV1) and total lung capacity (TLC) (Body Box 500; Medisoft, USA), following the recommendations of the American Thoracic Society and the European Respiratory Society (ATS / ERS) [6].
Measurement of exhalation NO
Exhaled NO was measured by using electrochemiluminescent technique with Hypair+NO (Medisoft; USA) as recommended by ATS/ERS [4].
Briefly, each patient after maximum inhaling from the ambient air blowed into the machine at a exhalation flow rate (EFR) of 50, 100, 150 or 300 mL/sec. Corresponding to each level of flow, the speed of exhaled NO was estimated by linear method.
Thus, FENO is inversely proportional to the exhalation flow rate and the speed of exhaled NO is a number function varying by the exhaled flow rate. For each patient and control group, the quality of the measurement was assessed by the R² correlation between FENO and expiratory flow rate and the value of R²> 0.90 was acceptable.
Patients who were unable to sustain exhalation within 8 seconds and failed to achieve an end-expiratory volume of at least 3 seconds were excluded from the study [4].
Polysomnography
All polysomnography were done for overnight sleep in the Sleep Lab Center of LMC, using the Alice PDx (Phillip; USA) with the electrodes and sensors recommended by the American Sleep Medicine Association (AASM). Four channels of electroencephalography were performed at positions of A2-C4, C4-C3, C3-A1 and C3-O1, 2 channels of electrooculography in the front and lower chin. Chest-abdominal movement amplitude, oral heat oscillates, nasal flow, saturation of peripheral oxygen (SpO2) was recorded continuously. Apnea was defined as total nasal–oral flow blocking for at least 10 seconds. Hypopnea was defined as a decrease of airflow (≥ 50%) that lasts for at least 10 seconds or a moderate decrease (<50%) accompanied by wakefulness on EEG and/or reduction of SpO2 at least 3% more than before (average SpO2 in 2 minutes ago). OSAS patients (AHI ≥5/hour) were divided into two groups: mild - moderate (AHI from 5 to 30/hour) and severe (AHI ≥30/hour). Patients with AHI <5/ hour were included in the control group. We assessed the reduction in oxygen saturation at night based on average SpO2, nadir SpO2 and sleep time with SpO2 <90% compared to sleep total (ST-90%). The standard for reducing blood oxygen saturation was ST-90% >1% of sleep time [5].
Statistical analyses
Data were analyzed, using SPSS software, version 16.0 (Chicago, Illinois, USA). Values are expressed as mean ±SD (standard deviations) for quantitative variables, in quantities and percentages for qualitative variables, except for exceptions that was be annotated separately. For continuous variables, the standard distribution was be tested by the KolmogorovSmirnov test.
The comparisons was conducted appropriately according to the Student's t-test or the Mann-Whitney test for quantitative variables and Chi-square test or Fisher's exact test for qualitative variables. The linear correlations between the NO parameters and the AHI or the reduction of blood oxygen saturation (ST90%) were performed using the Spearman nonparametric method. The statistical significance was p <0.05 with a 2-tailed test.
RESULTATS
From the beginning of January 2017 to the end of December 2017, 116 study participants were included and divided into 2 groups, 32 people in the control group (20 men, mean age of 44±13) and 84 OSAS patients (56 men, mean age of 59±11).
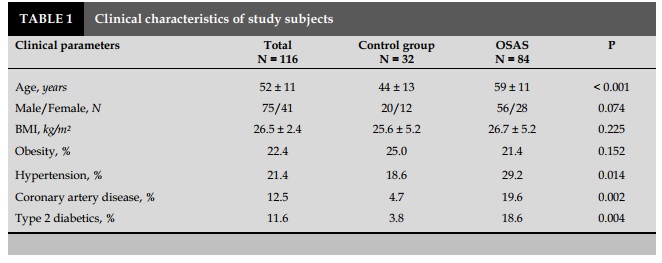
Characteristics of study subjects (Tables 1 and 2) Clinical characteristics and population function of the study are presented in Table 1 and 2. OSAS patients had a higher proportion of men than women and were older than the control group (P<0.05 and <0.001).
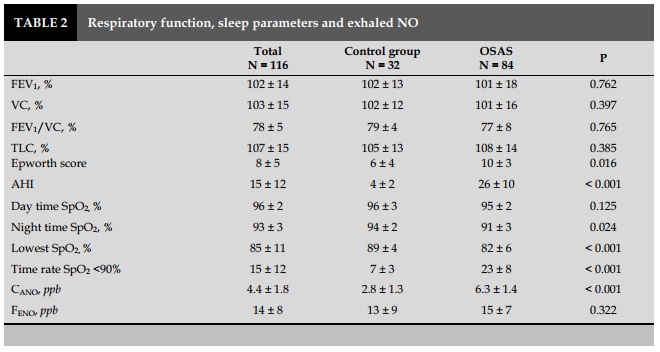
OSAS patients had an Epworth score (daytime drowsiness index) higher than that in the control group (P=0.016). AHI in patient group was 26±10/ hour, compared with the control group 4±2/ hour (P<0.001). The rate of sleep time with SpO2 was below 90% in patients with OSAS was higher than that in the control group (P<0.001; Table 2).
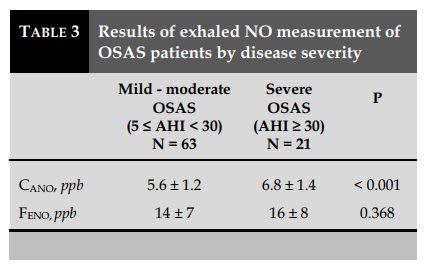
Exhaled NO and the severity of OSAS (Tables 2 and Table 3)
Average CANO of OSAS patients were (6.3±1.4 ppb), higher with statistical significance (P<0.001) as compared to the control group (2.8±1.3 ppb). However, there was no significant difference in J'awNO and total NO concentrations with 50 mL/sec exhalation flow (FENO) between the two groups (P>0.05) (Table 2). The results were similar to that of the non-OSAS group with the control group.
Severe OSAS patients (AHI ≥ 30/h, N=21) had significantly higher CANO (6.8±1.4 ppb) than those with mild to moderate OSAS (5.6±1.2 ppb). There was no significant difference between the two groups in J’awNO (data not showed) or in FENO (P>0.05) (Table 3).
The positive and linear correlations were statistically significant between CANO and AHI in OSAS patients (P<0.01; R=0.643; Figure 1). The correlation was not statistically significant in the group of patients without OSAS (data not showed).
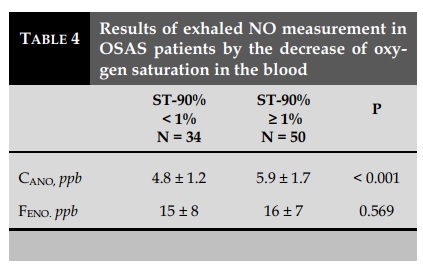
CANO and the decrease in SpO2
CANO in the group with SpO2 reduction (N=50; 5.9± 1.7 ppb) was significantly higher (p <0.001) than that in the group without SpO2 reduction (N=34; 4.8± 1.2 ppb). There was no significant difference between these two groups in J’awNO (data not showed) or FENO (P=0.569) (Table 4). There was a linear correlation with statistical significance (P<0.001; R=0.584) between CANO and ST-90% in patients with OSAS (AHI ≥ 5/hr; N=84) (Figure 2).
Correlation between CANO and other factors (age, obesity and hypertension)
There was no significant correlation between CANO, J’awNO or FENO.50 with age in OSAS patients and in control (P> 0.05). In the OSAS group, CANO was positively correlated with body mass index BMI (P= 0.012 and R=0.321). The correlation was not found in the control group (P=0.34). Multivariate regression analysis for CANO showed that this variable was significantly associated only with AHI (P<0.05 and β: 0.562±0.018), not associated with BMI (P=0.81). There was no significant correlation between J'awNO or FENO with BMI (P>0.05). There were no significant correlation in CANO, J’awNO or FENO among the OSAS patients with hypertension and those without hypertension (P> 0.05).
DISCUSSION
In this study, we demonstrated that the increased concentration of alveolar nitrogen oxide CANO were higher in patients with obstructive sleep apnea syndrome (OSAS) than those without OSAS and other people with no smoking. The increase of CANO was correlated with a decrease of AHI and sleep time rate with SpO2 <90%, suggesting that OSAS patients with increased CANO was associated with continuous hypoxia due to upper airway obstruction.
The cause of CANO increase is explained by the inflammation of the alveoli and distant airways and by endothelial cell dysfunction, the two main characteristics of OSAS patients [1-3]. Increased CANO caused by the airway inflammation has been demonstrated in several previous studies by the measurement of FENO, with the advantage of a simple and noninvasive test. This increase in NO was associated with an increase in NO synthase-2 (induced NOS) expression in neu- -trophils and macrophages from deep mucus in the airway [7] and the increase of oxidative stress indicators in exhaled breathing, which is condensed as hydrogen peroxide H2O2, 8-isoprostane, leukotriene B4 and nitrates [8]. The concentration of these indicators also increased significantly in plasma and was associated with AHI in OSAS patients [9]. These indicators are recovered partially after a period time of effective treatment with continuous positive pressure ventilation [8-11). This suggests that the phenomenon of CANO increase may be due to the blockage of the throat region in OSAS patients and may be clinically monitored by measuring exhaled NO [12]. Obesity is a risk factor for OSAS and is also one of the factors causing system inflammation through increased adipokines [1,2,13]. In our study, analyzing two-variable correlations showed a significant but weak correlation (R<0.5) between CANO and BMI, whereas with multivariate regression, this correlation does not exist anymore. This correlation may be disturbed by the AHI. This is consistent with the findings of Foresi and Fortuna, suggesting no statistically significant association between CANO and BMI [10,11]. However, other risk factors may be liked to OSAS in Vietnamese patients [14-23] and in different ethnics as presented previously [24-26]
CONCLUSION
The study demonstrated that OSAS patients had increased CANO and were associated with severe disease and hypoxia at night. This finding suggests that CANO measurements in breathing air may be used to screen for patients with high OSAS risk and hypoxemia at night among those with suspected OSAS symptoms. It is therefore preferable to select patients who require polysomnography because of its high cost for emergent countries.
CONFLIT OF INTERESTS
Non.
REFERENCES
1. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005; 112: 2660–7.
2. Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J 2009; 33: 1467–84.
3. Barnes PJ, Dweik RA, Gelb AF, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 2010; 138: 682–92.
4. American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 2005; 171: 912–30.
5. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5: 263-76.
6. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–38.
7. Depalo A, Carpagnano GE, Spanevello A, et al. Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med 2008; 263: 70–8.
8. Petrosyan M, Perraki E, Simoes D, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath 2008; 12: 207–15.
9. Carpagnano GE, Kharitonov SA, Resta O, et al. 8Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 2003; 124: 1386-92.
10. Foresi A, Leone C, Olivieri D, et al. Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest 2007; 132: 860–7.
11. Fortuna AM, Miralda R, Calaf N, et al. Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir Med 2011; 105: 630-6. 12. Carpagnano GE, Lacedonia D, Foschino-Barbaro MP. Non-invasive study of airways inflammation in sleep apnea patients. Sleep Med Rev 2011; 15: 317-26.
13. Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: A review and perspective. Sleep 2009; 32: 447-70.
14. Dang Thi Mai K, Tran Van N. Study of the prevalence of metabolic syndrome in patients with sleep apnea syndrome. J Func Vent Pulm 2013;04(10):36-42.
15. Nguyen Xuan Bich H. Obstructive sleep apnea syndrome (OSAS) and arterial hypertension. J Func Vent Pulm2014;05(14):1-2.
16. Hua-Huy T. Obstructive sleep apnoea syndrome (OSAS): on the right method for screening . J Func Vent Pulm 2014;05(15):3-4.
17. Duong-Quy S, Dang Thi Mai K, Tran Van N, et al. Study about the prevalence of the obstructive sleep apnoea syndrome in Vietnam. Rev Mal Respir. 2018 Jan;35(1):14-24.
18. Ho-Viet T.D, Soyez F. Role of mandibular advancement devices in management of obstructive sleep apnea syndrome (OSAS). J Func Vent Pulm 2015;06 (17):2-3.
19. Nguyen Thi Thanh P, Mai Thi Thanh T, Kim Xuan L. The quality of sleep in patients with COPD in Pham Ngoc Thach hospital - HCM city. J Func Vent Pulm 2015;19(6):44-48.
20. Duong-Quy S. Sleep disorder in COPD: a forgotten entity. J Func Vent Pulm 2015;19(6):1.
21. Martin F, Duong-Quy S. Sleep apnea syndrome in daily practice: Welcome to the 2nd edition of sleep disorder book in French - Vietnamese languages. J Func Vent Pulm 2016; 21(7): 1-2.
22. Nguyen-Thi-Hong L, Duong-Quy S. Obstructive Sleep Apnea Syndrome: The challenges in developing countries. J Func Vent Pulm 2016;22(7):1-2.
23. Nguyen-Hoang Y, Nguyen-Thi B. Laboratory techniques for diagnosis of obstructive sleep apnea (OSA) in children. J Func Vent Pulm 2018;27(9):2-10.
24. A d a m b o u n o u . A S , A d j o h . K S , Aziagbé. KA, Foma. W, Gbadamassi. AG, Tougan. A, Djibril. M, Belo. M, pémissi. E, Tidjani. O; Prevalence of symptoms of sleep apnea syndrome in Lome. J Func Vent Pulm 2016; 22(7):32-39.
25. A d j o h . K S , A d a m b o u n o u . A S , Gbadamassi. AG,Efalou. P, Ouedraogo. AR, Aziagbe. KA, Foma. W, Dolou. W, . Dijibril. MA, Belo. M. J Func Vent Pulm 2017; 24(8): 10-17.
26. Bacha S, Habibech S , Chaouch N. Profil clinique du syndrome d’apnées obstructives du sommeil. J Func Vent Pulm 2017;24(8):18-23.
FIGURES - TABLES
REFERENCES
1. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005; 112: 2660–7.
2. Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J 2009; 33: 1467–84.
3. Barnes PJ, Dweik RA, Gelb AF, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 2010; 138: 682–92.
4. American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 2005; 171: 912–30.
5. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5: 263-76.
6. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–38.
7. Depalo A, Carpagnano GE, Spanevello A, et al. Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med 2008; 263: 70–8.
8. Petrosyan M, Perraki E, Simoes D, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath 2008; 12: 207–15.
9. Carpagnano GE, Kharitonov SA, Resta O, et al. 8Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 2003; 124: 1386-92.
10. Foresi A, Leone C, Olivieri D, et al. Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest 2007; 132: 860–7.
11. Fortuna AM, Miralda R, Calaf N, et al. Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir Med 2011; 105: 630-6. 12. Carpagnano GE, Lacedonia D, Foschino-Barbaro MP. Non-invasive study of airways inflammation in sleep apnea patients. Sleep Med Rev 2011; 15: 317-26.
13. Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: A review and perspective. Sleep 2009; 32: 447-70.
14. Dang Thi Mai K, Tran Van N. Study of the prevalence of metabolic syndrome in patients with sleep apnea syndrome. J Func Vent Pulm 2013;04(10):36-42.
15. Nguyen Xuan Bich H. Obstructive sleep apnea syndrome (OSAS) and arterial hypertension. J Func Vent Pulm2014;05(14):1-2.
16. Hua-Huy T. Obstructive sleep apnoea syndrome (OSAS): on the right method for screening . J Func Vent Pulm 2014;05(15):3-4.
17. Duong-Quy S, Dang Thi Mai K, Tran Van N, et al. Study about the prevalence of the obstructive sleep apnoea syndrome in Vietnam. Rev Mal Respir. 2018 Jan;35(1):14-24.
18. Ho-Viet T.D, Soyez F. Role of mandibular advancement devices in management of obstructive sleep apnea syndrome (OSAS). J Func Vent Pulm 2015;06 (17):2-3.
19. Nguyen Thi Thanh P, Mai Thi Thanh T, Kim Xuan L. The quality of sleep in patients with COPD in Pham Ngoc Thach hospital - HCM city. J Func Vent Pulm 2015;19(6):44-48.
20. Duong-Quy S. Sleep disorder in COPD: a forgotten entity. J Func Vent Pulm 2015;19(6):1.
21. Martin F, Duong-Quy S. Sleep apnea syndrome in daily practice: Welcome to the 2nd edition of sleep disorder book in French - Vietnamese languages. J Func Vent Pulm 2016; 21(7): 1-2.
22. Nguyen-Thi-Hong L, Duong-Quy S. Obstructive Sleep Apnea Syndrome: The challenges in developing countries. J Func Vent Pulm 2016;22(7):1-2.
23. Nguyen-Hoang Y, Nguyen-Thi B. Laboratory techniques for diagnosis of obstructive sleep apnea (OSA) in children. J Func Vent Pulm 2018;27(9):2-10.
24. A d a m b o u n o u . A S , A d j o h . K S , Aziagbé. KA, Foma. W, Gbadamassi. AG, Tougan. A, Djibril. M, Belo. M, pémissi. E, Tidjani. O; Prevalence of symptoms of sleep apnea syndrome in Lome. J Func Vent Pulm 2016; 22(7):32-39.
25. A d j o h . K S , A d a m b o u n o u . A S , Gbadamassi. AG,Efalou. P, Ouedraogo. AR, Aziagbe. KA, Foma. W, Dolou. W, . Dijibril. MA, Belo. M. J Func Vent Pulm 2017; 24(8): 10-17.
26. Bacha S, Habibech S , Chaouch N. Profil clinique du syndrome d’apnées obstructives du sommeil. J Func Vent Pulm 2017;24(8):18-23.
ARTICLE INFO
DOI: 10.12699/jfvpulm.9.29.2018.32
Conflict of Interest
Non
Date of manuscript receiving
16/5/2018
Date of publication after correction
15/12/2018
Article citation
Minh Chau Luu, Tran-Thi-Khanh T, Duong Quy S. Increase of alveolar nitric oxide concentration in patients with sleep apnea and its correlation with nocturnal hypoxemia and sleep apnea severity. J Func Vent Pulm 2018;29(9):32-37.