
 English
English
 French
French
Obstructive Sleep Apnea (OSA) and Metabolic Syndrome (MetS)
Apnée Obstructive du Sommeil (AOS) et Syndrome Métabolique (SMet)
K. Dang Thi Mai1, T. Dang Vu1, N. Tran Van1, A. Nguyen Thi Hong1, S Duong – Quy2
1 : Respiratory Department. Cho Ray Hospital. Hochiminh city. Vietnam
2 : Lam Dong Medical College. Lam Dong, Da Lat, Vietnam
3 : Penn State Medical College. Hershey Medical Center. USA
Corresponding author
Dr. Dang Thi Mai Khue
MSc. Respiratory Department Cho Ray Hospital. HCM city. Vietnam.
E-mail: maikhuemd@gmail.com
ABSTRACT
Introduction. The aims of this study were to find out the prevalence of OSA in patients with metabolic syndrome and to highlight the importance of assessment of MetS in these patients.
Method. This cross-sectional analytical study was conducted on 164 subjects, comprising 23 cases of non-OSA and 141 cases with OSA. Overnight respiratory polygraphy was done in all the subjects. Prevalence of MetS was assessed and compared between the two groups.
Result. Prevalence of MetS was significantly higher (63.4%) in patients with OSA than in subjects without OSA (34.8%). There were 88 (91.7%) OSA patients with metabolic syndrome, 7 (8%) had mild OSA, 17 (19%) had moderate OSA, and 14 (73%) had severe OSA. Increasing severity of OSA was associated with MetS variables excluded HDL-c and Triglyceride.
Conclusion. OSA is shown to be associated with MetS, although causality between these two factors probably has not been demonstrated yet. Future cohort and randomized controlled studies are needed.
KEYWORDS: Obstructive sleep apnea; OSA; Metabolic syndrome; MetS; HDL-c; Triglyceride
RÉSUMÉ
Introduction. Les objectifs de cette étude étaient de découvrir la prévalence de l'AOS chez les patients atteints du syndrome métabolique et de souligner l'importance de l'évaluation du SMet chez ces patients.
Méthode. Cette étude analytique transversale a été menée sur 164 sujets, dont 23 cas de non-AOS et 141 cas avec AOS. Une polygraphie respiratoire nocturne a été réalisée chez tous les sujets. La prévalence du metS a été évaluée et comparée entre les deux groupes.
Résultat. La prévalence du SMet était significativement plus élevée (63,4%) chez les patients avec AOS que chez les sujets sans OSA (34,8%). Sur 88 (91,7%) patients souffrant d'AOS présentant un syndrome métabolique, 7 (8%) avaient une AOS légère, 17 (19%) une AOS modérée et 14 (73%) une AOS sévère. L'augmentation de la gravité de l'AOS était associée aux
variables SMet exclues HDL-c et triglycéride.
Conclusion. Il est démontré que l'AOS est associée au SMet, bien que la causalité entre ces deux facteurs n'ait probablement pas encore été démontrée. De futures études de cohorte et contrôlées randomisées sont nécessaires.
MOTS CLÉS: Apnée obstructive du sommeil; AOS; Syndrome métabolique; SMet; HDL-c; Triglycéride
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repeated episodes of apnea or shortness of sleep during sleep, resulting in fragmented sleep and intermittent hypoxia. The prevalence of OSA is about 13% for men and 6% for women [15], compared to 8.5% in Vietnam [8]. Obesity is considered to be one of the important risk factors for OSA. Obesity causes an increase in the size of the soft tissue in the oropharynx, thereby causing narrowing of the upper respiratory tract [10], [18].
The metabolic "non-OSA" association (MetS) is known to be associated with obesity, with "nonOSA" a high proportion of MetS in obese patients.
The incidence of MetS in Vietnamese is 12.3% [4].
On the other hand, OSA is considered a risk factor for cardiovascular diseases such as hypertension, heart failure, myocardial infarction or stroke [17].
OSA patients are also more likely to have diabetes or insulin resistance than normal people [13]. Since 1998 Wilcox et al. proposed the name "Association" nonOSA "Z" to describe the co-occurrence of OSA and MetS because both have the same risk factors as obesity and the same factors of cardiovascular disease, insulin resistance.
Recognizing OSA in patients with MetS and treating it has an important role in the comprehensive control of patient risk factors. This is also important for physicians to identify and refer patients to specialty clinics.
In this study, we want to investigate the incidence of MetS in patients with OSA and find out the correlation between OSA and MetS.
METHODS
The study was carried out in the Respiratory Department at Cho Ray Hospital and took samples from 2017-2019, including 2 groups of 23 "non-OSA" patients and 141 patients with OSA.
Criteria for selecting the study patients: adult patients came to do respiratory polygraphy in Respiratory Department of Cho Ray Hospital.
Patients with biochemical tests include: fasting blood glucose, LDL-c, HDL-c, Triglyceride, with all parameters: morning blood pressure, neck and waist circumference measurement.
Exclusion criteria: patients did not agree to participate in the study.
Research protocol: the study complied with the Helsinki Declaration. All patients in the study were explained and signed to participate in the study. All patients were given respiratory polygraphy overnight at the Respiratory Department of Cho Ray Hospital, including sensors: nasal flow sensor, thoracic movement, posture, abdominal movement, oxygen saturation and heart rate. The patient had blood pressure taken and a blood test in the next morning.
The recorded parameters of the respiratory polygraphy results including apnea-hypopnea index (AHI), SpO2 by CIDELEC 2.0 software and classify by standard recommendations of AASM (American Academy of Sleep Medicine) 2012. Apnea-Hypopnea Index (AHI) was used to assess OSA severity including: non-OSA ("non-OSA") with AHI <5 times/hour, 5-14 times /hour: mild; 15-29 times /hour: moderate; ≥ 30 times /hour: severe OSA.
Statistical analysis
The descriptive parameters of the study population characteristics, the identification variables and the order were presented as the number of objects (n) and the percentage (%); continuous variables were presented as mean, median and standard deviation (± SD). If the distribution of these variables was standard, Student t-test was chosen to compare the average between the two groups and the ANOVA test to compare the average of 3 or more groups. If the distribution was not normal distribution, a nonparametric test was used to compare the median with Mann-Whitney U test or Kruskal Wallis test for two groups or for three comparison groups or more.
RESULT
In our study, 164 subjects included: 124 males and 40 females; 23 (16 male) patients were non-OSA group and 141 (108 male) patients were OSA group. The gender distribution in the two groups was similar.
Among the study patients, 7 (4%) have the age group <30; 30-50 years: 59 (36%); 50-70 years old: 70 (43%); ≥ 70 years: 26 (16%). The mean age of the "non -OSA" group was 55.8 ± 17.6, of the OSA group: 53.5 ± 14.8, there was no significant difference. In particular, in the age group of 50-70 years, the OSA group was more significant than the "non-OSA" group (p <0.001).
The average circumference of the waist for the "nonOSA" group was 86.6 ± 8.5 cm, for the OSA group was 102.5 ± 12.1cm (p <0.001). The mean systolic and diastolic blood pressure of the "non-OSA" group and the OSA group were 117.8 ± 12.4 mmHg, 70.4 ± 10.2 mmHg and 125.9 ± 14.8 mmHg, 76.7 ± 11.4 mmHg, respectively (p <0.05).
The fasting blood glucose of the "non-OSA" group and the OSA group were 102.0 ± 22.3 and 107.4 ± 40.3 mg%, respectively (p >0.05). The average Triglycerides of non-OSA and OSA groups were 188.8 ± 116.3 and 239.4 ± 346mg% (p >0.05). HDL-c of the "non-OSA" group and the OSA group were, respectively, 41.7 ± 12.6 and 50.6 ± 71.6 mg% (p >0.05).
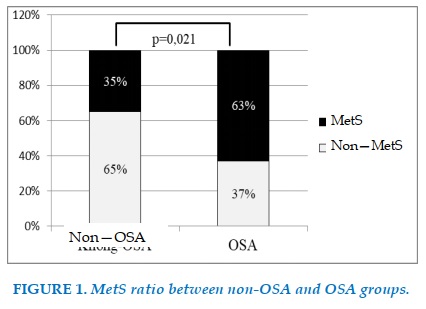
Of the 23 "non-OSA" group subjects, 8 (34.8%) had MetS, while 141 OSA group subjects had 88 (63.4%) with MetS (Figure 1). This difference was statistically significant (p = 0.021).
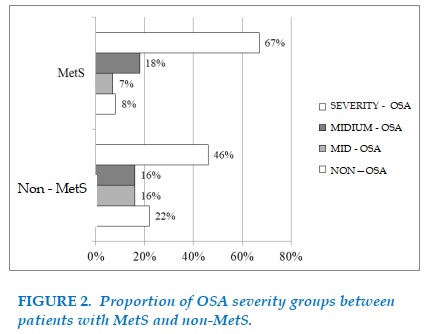
96 patients (58.5%) of the study subjects had MetS, of which 74 patients (45%) had hypertension, 99 patients (60%) increased Triglycerides, 67 patients (41%) had hyperglycemia fasting and 90 patients (55%) with HDL-c increase. Among 164 patients, 96 patients (58%) had MetS and 68 patients (42%) were non-MetS. Among 68 patients in the "non-MetS" group, there were 15 patients (22%) with no -OSA, 11 patients. patients (16%) had mild OSA, 11 patients (16%) average OSA, 31 patients (46%) severe OSA. Of the 96 patients with MetS, there were 8 patients (8%) with no –OSA, 7 patients (7%) with mild OSA, 17 patients (18%) with average OSA, 64 patients (67%) with severe OSA. This difference is statistically significant (p = 0.010) (Figure 2).
The average AHI in the MetS group was 47.1 ± 29.0 times /hour, while the AHI of the non-MetS group was 31.7 ± 27.3 times /hour. This difference is statistically significant (p = 0.001).
Among 124 men, 67 patients (46%) had MetS and 57 patients (54%) were non-MetS. Among men with MetS, 5 patients (8%) were non-OSA, 3 patients (5%) had mild OSA, 8 patients (12%) average OSA, 51 patients (76%) had severe OSA. Among non-MetS men, 11 patients (19%) were non-OSA, 8 patients (14%) had mild OSA, 9 patients (16%) average OSA, 29 patients (51%) had severe OSA. This difference is statistically significant (p = 0.002).
Among 40 females, 11 patients (27.5%) were nonMetS and 29 patients (72.5%) had MetS. Among women with MetS, 3 patients (10%) were non-OSA, 4 patients (14%) had mild OSA, 9 patients (31%) average OSA, 13 patients (45%) had severe OSA. Among non-MetS females, 4 patients (36%) were non-OSA, 3 patients (27%) had mild OSA, 2 patients (18%) had moderate OSA, 2 patients (18%) had severe OSA. This difference is not statistically significant (p =0.116).
The percentage of men with MetS was significantly higher than females (p = 0.039).
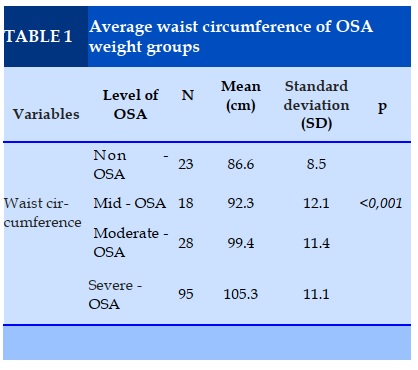
The average waist circumference of the "non-OSA" group and the mild OSA, average OSA, and heavy OSA were 86.6 ± 8.5, 92.3 ± 12, 99.4 ± 11.4, 105.3 ± 11.1 cm. This difference is statistically significant (p<0.001) (Table 1).
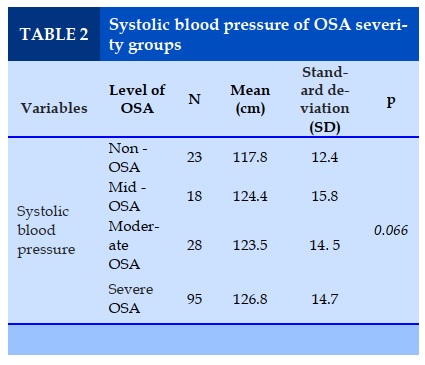
The systolic blood pressure of the OSA group (125.9 ± 14.8 mmHg) was significantly higher than the "non-OSA" group (117.8 ± 12.4 mmHg) (p = 0.014). However, when comparing the "non-OSA" and the light OSA, average OSA, OSA weighs 117.8 ± 12.4, respectively; 124.4 ± 15.8; 123,6 ± 14.5; 126.8 ± 14.7 mmHg. This difference is not statistically significant (Table 2).
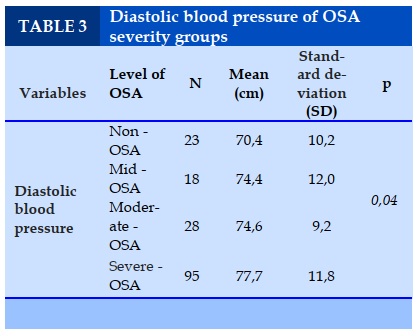
Diastolic blood pressure of the "non-OSA" group and mild OSA, average OSA, OSA weight were 70.4 ±10.2 respectively; 74.4 ± 12.0; 74.6 ± 9.2; 77.7 ± 11.8 mmHg. This difference was statistically significant(Table 3).
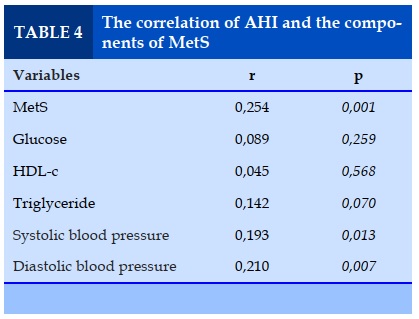
AHI had a positive, weak correlation with metabolic "non-OSA" and blood pressure index. AHI is not correlated with other indicators including blood sugar, triglycerides or blood cholesterol (Table 4).
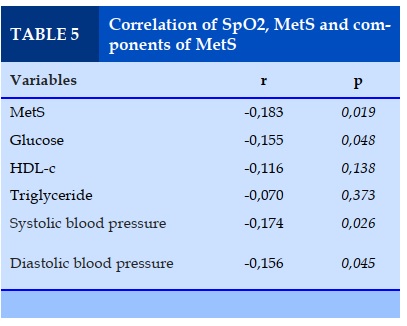
The lowest sleep SpO2 was inversely correlated with MetS and its components including blood sugar and blood pressure. The lowest SpO2 during sleep did not correlate with other variables such as Triglyceride or Cholesterol (Table 5).
DISCUSSION
Our study investigates the correlation between MetS and OSA. We focused on understanding the incidence of MetS in OSA patients and the proportion of OSA in patients with MetS was higher than the nonMetS group. On the other hand, we showed the correlation of metabolic disorders and parameters of multiple respiratory polygraphy.
The study involved 164 patients including the "nonOSA" group and the OSA group. The percentage of patients with MetS in the OSA group (63%) was significantly higher than that of the "non-OSA" group (35%) was statistically significant. Besides that, the AHI of MetS group (47.1 ± 29.0 times / hour) was significantly higher than the "non-MetS" group (31.7 ± 27.3 times /hour). The percentage of OSA patients in the MetS group (98%) was significantly higher than the "non-
MetS" group (78%). The previous study showed that the proportion of OSA among MetS patients was higher than that of the "non-MetS" group. "Non-MetS" [19], [20]. Perez’s
study involving 83 female patients, showed the incidence of MetS and OSA was 75.8% [16]. MetS characterized by central obesity is also a risk factor for OSA, increasing the load on the upper respiratory tract, causing narrowing and collapse of the airway. Fat is concentrated under the jaw and under the tongue, the soft palate, and the tongue. Obesity also causes a decrease in lung volume during sleep due to a combination of increased fat and supine position, which reduces the pressure causing collapse along the trachea and reducing the pressure on the larynx; it contributes to the upper airway narrowing.
Compared with peripheral obesity, central obesity increased the fat volume in the neck more significantly causing airway narrowing during sleep [9].
In our study, men with "non-OSA" metabolic rates was significantly higher than females. Many studies also show possible gender differences in MetS [12], [14]. The waist circumference of the OSA group was significantly higher than the "non-OSA" group. In another study by Suryakumari, similar results were found with mean waist circumference of the OSA group (110.9 cm) significantly higher than the nonOSA group (98.1 cm) (p = 0.01) [19]. Increased waist circumference in OSA patients showed correlation of OSA and central obesity [7].
The systolic and diastolic blood pressure of the OSA group was significantly higher than the "non-OSA" group. However, when comparing different weight groups, only diastolic blood pressure increased significantly. In Dubey et al's study, the diastolic blood pressure of the "non-OSA" group, light, medium, and heavy OSA were 88, 91.2, 93.9, and 97.8 mmHg, respectively [6]. Similarly, in our study, the systolic blood pressure of the OSA group (146.8 mmHg) was significantly higher than the non-OSA group (125 mm Hg) [19]. The Perez [16] and Walia [21] studies also show an association of OSA and resistant hypertension. Blood pressure in severe OSA patients is more difficult to control than in "non-OSA" patients [5].
Our study shows a correlation between blood pressure and OSA severity, specifically AHI. There are many mechanisms that can explain this correlation. Severe OSA causes endothelial function damage due to intermittent hypoxia during sleep, which causes oxidative stress. Endothelial damage is also the mechanism that causes hypertension [11]. More specifically, in our study, hypoxemia was negatively correlated with blood pressure index, supporting the hypothesis. OSA increases sympathetic activity that affects aldosterone secretion which plays a role in difficulty controlling blood pressure. OSA treatment helps control resistant high blood pressure [5]. We also conducted a similar study in 2012 [1] and also found a correlation with the lowest AHI and SpO2 during sleep. Study by Nguyen Ngoc Phuong Thu shows that the high rate of OSA in hypertensive patients and the treatment of OSA help reduce blood pressure [3]. Duong Quy Sy also showed a high proportion of OSA in hypertensive patients (84%) [2].
Although fasting blood glucose did not differ between OSA and "non-OSA" groups, it was correlated with hypoxia during sleep. Intermittent hypoxia during sleep causes oxidative stress, perhaps the mechanism that explains this fasting blood sugar disorder [22]. Sinem Nedime Sökücü's study [23] also showed that fasting blood sugar correlated with the lowest SpO2 during sleep with r = –0,368, P = 0.004.
In our study, no differences between HDL-c and Triglycerides were found between the "non-OSA" group and the OSA group. Our study shows the relationship of MetS and OSA as well as the severity of OSA relative to the components of MetS.
CONCLUSION
OSA is related to MetS although it seems that the cause of this is not certain, probably related to obesity. More cohort studies are needed to find out this causative mechanism.
CONFLIT OF INTEREST
Non.
REFERENCES
1. Dang Thi Mai Khue (2012), Survey on the prevalence of metabolic "non-OSA" in patients with sleep apnea Department of Internal Medicine, University of Medicine and Pharmacy, Ho Chi Minh City.
2. Duong Quy Sy. (2013), "Study on sleep apnea characteristics in hypertensive patients", Vietnam Journal of Medicine. 407 (1), pp. p 82-86.
3. Nguyen Ngoc Phuong Thu (2019), Frequency of sleep apnea in hypertensive patients and the effect of CPAP treatment on blood pressure, Department of Internal Medicine, University of Medicine and Pharmacy, Ho Chi Minh City.
4. Tran Quang Binh (2015), "Non-OSA" Association transformed in people with normal body mass index in the community of Ha Nam province ", Journal of Preventive Medicine. 15 (8), pp. 168.
5. Martínez-García M. A. et al. (2006), "Sleep-disordered breathing in patients with difficult-to-control hypertension", Archivos de bronconeumologia. 42 (1), 14-20.
6. AP D. (2017), "Prevalence of obstructive sleep apnoea in metabolic syndrome", Int J Adv Med. 4 (3), pp. 722-727.
7. Castaneda A. et al. (2018), "Correlation between metabolic syndrome and sleep apnea", World journal of diabetes. 9 (4), pp. 66-71.
8. Duong Quy S. et al. (2015), "EPSASIE : étude épidémiologique du syndrome d’apnées obstructives au cours du sommeil (SAOS) au Vietnam", Médecine du Sommeil. 12 (1), pp. 26-27.
9. Grundy S. M. et al. (2004), "Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition", Circulation. 109 (3), pp. 433-438.
10. Horner R. L. et al. (1989), "Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea", The Quarterly journal of medicine. 72 (268), pp. 719-735.
11. Hou H. et al. (2018), "Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis", Journal of global health. 8 (1), pp. 010405-010405.
12. Lee S. et al. (2016), "Gender differences in metabolic syndrome components among the Korean 66-year-old population with metabolic syndrome", BMC geriatrics. 16, pp. 27-27.
13. Muraki I. et al. (2018), "Sleep apnea and type 2 diabetes", Journal of diabetes investigation. 9 (5), pp. 991-997.
14. Park E. et al. (2015), "Gender- and age-specific prevalence of metabolic syndrome among Korean adults: analysis of the fifth Korean National Health and Nutrition Examination Survey", The Journal of cardiovascular nursing. 30 (3), pp. 256-266.
15. Peppard P. E. et al. (2013), "Increased prevalence of sleep-disordered breathing in adults", Am J Epidemiol. 177 (9), pp. 1006-1014.
16. Perez E. A. et al. (2017), "Prevalence and severity of syndrome Z in women with metabolic syndrome on waiting list for bariatric surgery: a cross-sectional study", Diabetology & metabolic syndrome. 9, pp. 72-72.
17. Ryan S. (2018), "Mechanisms of cardiovascular disease in obstructive sleep apnoea", Journal of Thoracic Disease. 10 (Suppl 34), pp. S4201-S4211.
18. Shelton K. E. et al. (1993), "Pharyngeal fat in obstructive sleep apnea", The American review of respiratory disease. 148 (2), pp. 462-466.
19. Suryakumari V. (2018), "Study of Prevalence of Obstructive Sleep Apnea in Patients With Metabolic Syndrome", Journal of Dental and Medical Sciences. 17 (1), pp. 51-55.
20. Venkateswaran S. et al. (2007), "The prevalence of syndrome Z (the interaction of obstructive sleep apnoea with the metabolic syndrome) in a teaching hospital in Singapore", Postgraduate medical journal. 83 (979), pp. 329-331.
21. Walia H. K. et al. (2014), "Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use", Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 10 (8), pp. 835-843.
22. Yokoe T. et al. (2008), "Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice", J Physiol. 586 (3), pp. 899-911.
23. Sökücü S. N. et al. (2013), "Effect of Hypoxia on Glucose Metabolism in Nondiabetic Patients With Obstructive Sleep Apnea Syndrome", Archivos de Bronconeumología (English Edition). 49 (8), pp. 321-325.
REFERENCES
1. Dang Thi Mai Khue (2012), Survey on the prevalence of metabolic "non-OSA" in patients with sleep apnea Department of Internal Medicine, University of Medicine and Pharmacy, Ho Chi Minh City.
2. Duong Quy Sy. (2013), "Study on sleep apnea characteristics in hypertensive patients", Vietnam Journal of Medicine. 407 (1), pp. p 82-86.
3. Nguyen Ngoc Phuong Thu (2019), Frequency of sleep apnea in hypertensive patients and the effect of CPAP treatment on blood pressure, Department of Internal Medicine, University of Medicine and Pharmacy, Ho Chi Minh City.
4. Tran Quang Binh (2015), "Non-OSA" Association transformed in people with normal body mass index in the community of Ha Nam province ", Journal of Preventive Medicine. 15 (8), pp. 168.
5. Martínez-García M. A. et al. (2006), "Sleep-disordered breathing in patients with difficult-to-control hypertension", Archivos de bronconeumologia. 42 (1), 14-20.
6. AP D. (2017), "Prevalence of obstructive sleep apnoea in metabolic syndrome", Int J Adv Med. 4 (3), pp. 722-727.
7. Castaneda A. et al. (2018), "Correlation between metabolic syndrome and sleep apnea", World journal of diabetes. 9 (4), pp. 66-71.
8. Duong Quy S. et al. (2015), "EPSASIE : étude épidémiologique du syndrome d’apnées obstructives au cours du sommeil (SAOS) au Vietnam", Médecine du Sommeil. 12 (1), pp. 26-27.
9. Grundy S. M. et al. (2004), "Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition", Circulation. 109 (3), pp. 433-438.
10. Horner R. L. et al. (1989), "Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea", The Quarterly journal of medicine. 72 (268), pp. 719-735.
11. Hou H. et al. (2018), "Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis", Journal of global health. 8 (1), pp. 010405-010405.
12. Lee S. et al. (2016), "Gender differences in metabolic syndrome components among the Korean 66-year-old population with metabolic syndrome", BMC geriatrics. 16, pp. 27-27.
13. Muraki I. et al. (2018), "Sleep apnea and type 2 diabetes", Journal of diabetes investigation. 9 (5), pp. 991-997.
14. Park E. et al. (2015), "Gender- and age-specific prevalence of metabolic syndrome among Korean adults: analysis of the fifth Korean National Health and Nutrition Examination Survey", The Journal of cardiovascular nursing. 30 (3), pp. 256-266.
15. Peppard P. E. et al. (2013), "Increased prevalence of sleep-disordered breathing in adults", Am J Epidemiol. 177 (9), pp. 1006-1014.
16. Perez E. A. et al. (2017), "Prevalence and severity of syndrome Z in women with metabolic syndrome on waiting list for bariatric surgery: a cross-sectional study", Diabetology & metabolic syndrome. 9, pp. 72-72.
17. Ryan S. (2018), "Mechanisms of cardiovascular disease in obstructive sleep apnoea", Journal of Thoracic Disease. 10 (Suppl 34), pp. S4201-S4211.
18. Shelton K. E. et al. (1993), "Pharyngeal fat in obstructive sleep apnea", The American review of respiratory disease. 148 (2), pp. 462-466.
19. Suryakumari V. (2018), "Study of Prevalence of Obstructive Sleep Apnea in Patients With Metabolic Syndrome", Journal of Dental and Medical Sciences. 17 (1), pp. 51-55.
20. Venkateswaran S. et al. (2007), "The prevalence of syndrome Z (the interaction of obstructive sleep apnoea with the metabolic syndrome) in a teaching hospital in Singapore", Postgraduate medical journal. 83 (979), pp. 329-331.
21. Walia H. K. et al. (2014), "Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use", Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 10 (8), pp. 835-843.
22. Yokoe T. et al. (2008), "Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice", J Physiol. 586 (3), pp. 899-911.
23. Sökücü S. N. et al. (2013), "Effect of Hypoxia on Glucose Metabolism in Nondiabetic Patients With Obstructive Sleep Apnea Syndrome", Archivos de Bronconeumología (English Edition). 49 (8), pp. 321-325.
ARTICLE INFO DOI: 10.12699/jfvpulm.10.30.2020.36 Conflict of Interest