
 English
English
 French
French
Association of sleep-related breathing disorders with asthma control in children
Association des troubles respiratoires liés au sommeil au contrôle de l'asthme chez les enfants
Nguyen-Ngoc-Quynh1, L. Luong-Thi1, C. Le-Quynh1, D. Tran-Minh1, M. Nguyen-Thi-Thanh2, T. Nguyen-Thi-Dieu2, S. Duong-Quy3,4,5
1: Department of Immuno-Allergology and Asthma. National Children’s Hospital. Hanoi, Vietnam
2: Department of Pediatrics. Hanoi Medical University. Hanoi, Vietnam
3: Department of Respiratory Diseases. Lam Dong Medical College. Dalat, Vietnam
4: Division of Immuno-Allergology. Penn State Medical College. Hershey Medical Center. PA, USA
5: Pham Ngoc Thach University of Medicine. Ho Chi Minh City. Vietnam
11
Corresponding author:
DUONG-QUY. Lam Dong Medical College and Penn State Medical College. PA, USA
E-mail: sduongquy.jfvp@gmail.com
ABSTRACT
Introduction. Asthma is one of the most common chronic conditions in childhood and have linked to sleep-related breathing disorders (SRBD), both of these conditions share multiple epidemiological risk factors. Aim of this study was to examine the association between SRBD and asthma control in children with asthma.
Methods. Children with asthma will referred for assessment of FENO and pulmonary function test. Asthmatic children completed the Asthma Control Test (ACT) and Pediatric Sleep Questionnaire (PSQ). C-ACT score ≤ 19 are indicative of poor asthma control whereas PSQ scores ≥ 0.33 are sugggestive of high risk for OSA.
Results. 54 asthmatic children, mean age was 8.38 ± 2.38 years. High risk for SRBD was identified in 16 children with PSQ scores ≥ 0.33. A significant high risk of SRDB was found among children with poor controlled asthma (PSQ score 0.38±0.18 vs 0.18±0.13, p< 0.05). Among children with high risk, 81.25% were diagnosed with both asthma and allergic rhinitis.
Conclusions. SRBD is associated with poor asthma control. The presence of allergic rhinitis in children with asthma seems to increase the prevalence of SRDB in that population.
KEYWORDS: Sleep-related breathing disorder; SRBD; Asthma; ACT; PSQ; C-ACT.
RÉSUMÉ
Introduction. L'asthme est l'une des affections chroniques les plus courantes chez l'enfant et est lié aux troubles respiratoires liés au sommeil (SRBD), ces deux affections partagent de multiples facteurs de risque épidémiologiques. Le but de cette étude était d'examiner l'association entre le SRBD et le contrôle de l'asthme chez les enfants asthmatiques.
Méthodes. Les enfants asthmatiques seront référés pour une évaluation du FENO et un test de la fonction pulmonaire. Les enfants asthmatiques ont rempli le test de contrôle de l'asthme (ACT) et le questionnaire sur le sommeil pédiatrique (PSQ). Un score C-ACT ≤ 19 indique un mauvais contrôle de l'asthme, tandis que des scores PSQ ≥ 0,33 suggèrent un risque élevé d'AOS.
Résultats. 54 enfants asthmatiques, l'âge moyen était de 8,38 ± 2,38 ans. Un risque élevé de SRBD a été identifié chez 16 enfants avec des scores PSQ ≥ 0,33. Un risque élevé significatif de SRDB a été trouvé chez les enfants dont l'asthme était mal contrôlé (score PSQ 0,38 ± 0,18 vs 0,18 ± 0,13, p < 0,05). Parmi les enfants à haut risque, 81,25 % ont reçu un diagnostic d'asthme et de rhinite allergique.
Conclusions. La SRBD est associée à un mauvais contrôle de l'asthme. La présence de rhinite allergique chez les enfants asthmatiques semble augmenter la prévalence de la SRDB dans cette population.
MOTS CLÉS: Trouble respiratoire lié au sommeil; SRBD; Asthme; PSQ; C-ACT.
INTRODUCTION
Asthma is a chronic inflammatory disease of the airways, characterized by respiratory symptoms such as wheezing, dyspnea, chest tightness, and cough. The process of airway inflammation in bronchial asthma involves many inflammatory cells including mast cells, eosinophils, and Th2 lymphocytes [1]. According to the data of the World Health Organization, the prevalence of asthma in children is 10- 12%, tends to increase rapidly, especially in developing countries in the Asia-Pacific region [2].
Sleep-related breathing disorder (SRBD) affects 5.1- 13.3% of children, it ranges from primary snoring to obstructive sleep apnea syndrome [3,4]. It is now apparent that SRBD can lead to substantial morbidities affecting neurobehavioral morbidities, cardiovascular and metabolic systems, and somatic growth, ultimately leading to reduced quality of life [5]. Recently, an association between asthma and SRBD has been proposed. A systemic review of 17 studies that included 45,155 children showed that asthmatic children have 1.91 times higher odds of reporting symptoms that suggest SRBD [6].
The link among asthma and SRBD is bidirectional due to common risk contributors that induce airway inflammation [7]. SRBD in children with asthma may lead to difficult- to- control asthma after adjusting for other established factors to asthma control [8]. On the other hand, asthma is also associated with airway inflammation and resistance, which causes decreased airway flow rates during sleep, thereby affecting the quality of sleep [9].
Furthermore, common comorbidity of asthma such as obesity, gastroesophageal reflux and allergic rhinitis may also lead to SRDB due to narrowing of the upper airway by local fat infiltration [3], altered parasympathetic tone to trigger bronchoconstriction [10], and nasal congestion [11]. These mechanisms provide evidence that the prevalence of asthma is associated with a high risk of SRBD in children.
Pediatric Sleep Questionnaire (PSQ) developed and validated by Chervin et al. is a 22-item questionnaire which had been shown to have a sensitivity of 81% and specificity of 87% for SRBD [12]. PSQ is one of the most popular parent-report scales for screening sleep problems in children aged between 2 and 18 in both clinical and research settings [13].
The questionnaire, divided into 10 sections, investigates different aspects of the quality of the sleep, from snoring to diurnal and nocturnal behavioural habits. According to European Respiratory Society Task Force, PSQ is defined as a “useful tool” to predict
obstructive sleep apnea syndrome, with an apnoea–hypopnea index (AHI) of >5, detecting the neurobehavioral consequences associated with obstructive sleep apnea syndrome and/or evaluating their regression after adenotonsillectomy, which is considered the first choice for obstructive sleep apnea syndrome treatment in children [14].
According to the Global Initiative for Asthma (GINA), asthma control is based on the frequency of symptoms, any night waking due to asthma or limitation of activity and frequency of reliever medication use [1]. Several asthma control tools have been developed for children and adults. Among these tools is the Asthma Control Test (ACT), a simple score, not using lung function test results, which has been validated for the assessment of asthma control especially in limited-resources settings [15].
Regarding children’s, the childhood Asthma Control Test (c- ACT) is in alignment with asthma guidelines and represents one of the most extensively validated prognostic modality tools [16]. FENO is also an important parameter of asthma management. A recent meta- analysis suggests that using FENO to guide treatment decisions may result in a lower rate of exacerbations [17].
The purpose of this study was to examine the assciation between SRBD risk and asthma control in asthmatic children in Vietnam.
METHODS
Subjects
From January 2021, we began to prospectively classify the level of asthma control among all children with the diagnosis of asthma being routinely followed at Immunology- Allergy- Rheumatology department, National Children’s hospital, Vietnam.
Inclusion criteria
All subjects aged between 6 and 17 years and fulfilling the criteria for a clinician- confirmed diagnosis of asthma as defined by Global Initiative for Asthma (GINA) 2020 or children over 5 years old,1 were included in the study.
Exclusion criteria
Subjects were excluded from the study if they had one of the following features: other significant chronic or acute diseases, facial structure malformation, mental disorders which made subjects unable to perform spirometry and ventilatory polygraphy.
Methods
Study design
It was a prospective analysis of all subjects with asthma at Immunology- Allergy- Rheumatology department, Vietnam National Children’s hospital after approved by Hanoi Medical University Institutional Ethical Review Board (IRB-VN01.001/IRB00003121/FWA 00004148).
Anthropometry
Children were weighed using a calibrated scale to the nearest 0.1 kg and height (to 0.1cm) was measured with a stadiometer (Medisol, Vietnam). Body mass index (BMI) was calculated and BMI z- score was computed using Baylor college of medicine Age- based Pediatric Growth Reference Charts (https: //www.bcm.edu / bodycomplab / BMIapp / BMI-calculator-kids.html) [18]. A BMI Percentile of 85th to less than the 95th percentile was considered as overweight and a BMI Percentile equal to or greater than the 95th percentile fulfilled the criteria for obesity [18].
Lung function testing (spirometry) was done by Koko (InSpire Health, Inc., Longmont, CO, USA). The reversibility of forced expiratory volume in 1 s (FEV1) was evaluated after using 200 μg of salbutamol for 15 min. The test was positive when there was an increase in FEV1 ≥12% and >200 Ml [1].
Measuring exhaled NO concentration was done by Hypair FeNO+ Device (Medisoft; Sorinnes, Belgium) with expiratory air flow of 50, 100, 150, 350 mL/s. Fractional exhaled nitric oxide (FENO) levels were classified as recommended by the American Thoracic Society/European Respiratory Society (ATS/ERS) for children (<20 ppb: normal; 20–35 ppb: increased; and >35 ppb: highly increased) [17].
Asthma Control Selection Criteria
The level of asthma control was measured using the Asthma Control Test (ACT) [15]. The ACT questionnaire assesses the symptoms of asthma in the last 4 weeks. Patients are asked to rate the following items: daily activity limitations, shortness of breath, nocturnal awakening and the use of rescue medications. They are also asked to rate their asthma control.
Each questionnaire with an ACT score ≥20 indicates controlled asthma, while 16 to 19 indicates partly controlled asthma and ≤15 characterizes poorly controlled asthma. In the current study, we considered patients with a score <20 as having inadequately controlled asthma. The Vietnamese version of ACT is validated in 2012 [19].
The c-ACT for children aged older than 4 and younger than 12 years, was complemented by parents and children with asthma. The c-ACT is divided into two separated parts; the first part is supplemented by the child and consists of four components.
The gradation of the responses from the first part ranges from 0 to 3.
The second part is answered by the escorting parent or guardian and consists of three other components ranging from 0 to 5. The total score of c-ACT is the sum of all responses, ranging from 0 which corresponds to the poorest asthma control, up to the value 27 which represents the optimal control of asthma. A value ≤19 indicates uncontrolled asthma [16].
Depending on asthma severity, the study subjects were treated as recommended by GINA (intermittent asthma: short-acting beta agonist [SABA] as needed; mild asthma: low-dose inhaled corticosteroid (ICS) + SABA; moderate asthma: moderate- to high-dose ICS + SABA; severe asthma: moderate- to high-dose ICS + long-acting beta agonist + SABA) [1].
PSQ questionnaire
The PSQ- SRBD questionnaire was filled out by parents. We have used a Vietnamese, non- validated version. Subscales within the SRBD scale include a 4-item sleepiness scale, a 4-item snoring scale, and a 6-item in attention/hyperactivity scale derived originally from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for attention-deficit/hyperactivity disorder (ADHD) [12].
Responses are “yes”=1, “no”=0, and “don’t know”=missing. The mean response on nonmissing items is the score, which can vary from 0 to 1. Previous data suggest that a cut-off value of 0.33 would be most effective in identifying pediatric SRBD.
Data Analysis
Statistical analyses were performed using SPSS software (version 22.0; IBM Corporation, Armonk, NY, USA). The t-test was used to analyse the differences between groups if the group variances were homogeneous, whereas the Mann–Whitney U test was used to analyse the differences between groups if the group variances were heterogeneous. Pearson's chi-squared test or Fisher's exact test was used to analyse categorical variables. Significance was defined at the 5% level (P < 0.05).
RESULTS
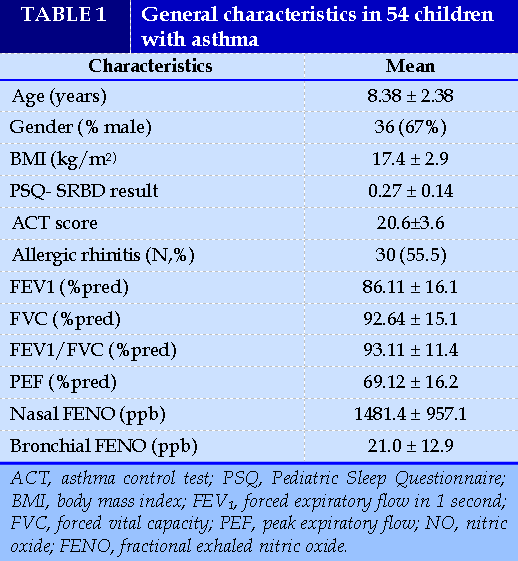
A total of 54 patient with asthma have been included in the study. Their demographic characteristics are shown in Table 1. The mean age was 8.38 ± 2.38 years (6–12 years) with 63% of male and 37% of female children. Only 9,5% of subject were obese. Approximately 55.5% of the study subjects had a history of allergic rhinitis.
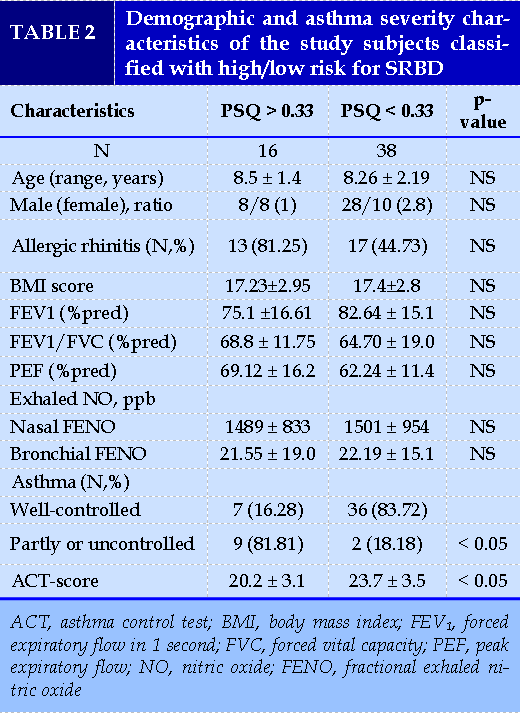
Sixteen children were identified as high risk for SRBD group (mean PSQ ± SD 0.4 ± 0.08). The percentage of children in high risk for SRBD in the whole group of participants was 29.6%. Comparison between these 16 children and children with normal PSQ score did not reveal statistically important differences in terms of age, gender, FENO value, or pulmonary function tests (Table 2).
We performed a statistical analysis (t- test), comparing children with poor asthma control (c-ACT ≤ 19; n = 11) with children with adequate/good control (c-ACT >19; n =43) in terms of PSQ-SRBD scores. The results were the following: In children with poor asthma control (c- ACT score = 18.2 ± 4.1): PSQ- SRBD 0.38 ± 0.18; in children with good asthma control (c-ACT score = 22.4 ± 3.6): PSQ- SRBD 0.18 ± 0.13 (p<0.05). Of note, total scores ≥ 0.33 are considered positive and suggestive of high risk for pediatric SRBD. This analysis indicated that in poor asthma control PSQ- SRBD values are higher and tend to show positive SRBD. A limitation in this analysis was the very small number, only 11, of children with c-ACT ≤19.
A statistically significant difference was noticed in mean values of c- ACT between the two subgroups (20.2 ± 3.1 vs. 23.7 ± 3.5; p <0.05), reporting a correlation between asthma control and less likelihood of SRBD.
In the subgroup of children with high risk for SRBD, the majority (n =13; 81.25%) were simultaneously diagnosed with asthma and allergic rhinitis, only three were suffering from asthma alone.
DISCUSSION
This study demonstrates a high prevalence of SRBD in patients with poor control asthma as compared to the prevalence of SRBD in the general population. In the present study, we found that the risk of SRBD in children with asthma was 29.6%. We also found that partly controlled or uncontrolled asthma was a risk factor for SRBD in children; coexistence of SRBD (diagnosed based on PSQ scores) was significantly more frequent in children with poorly controlled asthma than in those with well- controlled asthma. Our findings are concordant with those of previous studies. Several studies have shown that SRBD symptoms, such as snoring and witnessed apnea, are also common in the asthmatic population. A study of 408 children with asthma aged 4–18 years, children with poorly controlled asthma had a higher prevalence of SRBD than children with well-controlled asthma (59.6% vs. 18.2%) [8]. Another study evaluated 209 asthmatic children found that the risk of SRBD was significantly higher in children with poorly controlled asthma than in children with well- controlled asthma (34.25% vs 13.97%) [20].
Among the majority of children with high risk for SRBD both asthma and allergic rhinitis were observed, compared to children with low risk, where asthma without allergic rhinitis was more prevalent. Nasal obstruction, especially caused by allergic rhinitis, has been considered as one of the leading risk factors for upper airway obstruction during sleep, linked to SRBD, including snoring and obstructive sleep apnea [21]. The role of nasal congestion in increasing the risk of SRBD has been confirmed by previous studies in sleep-related problems with the nasal occlusion model [22]. However, until now, the exact mechanism for the link between allergic rhinitis and OSA has not been clearly identified [23-26]. In the present study, we found that 55.5% asthmatic children had allergic rhinitis. The prevalence of allergic rhinitis was not different between the children with and without SRBD.
There was no relationship between the clinical asthma characteristics and the presence of SRBD. Previous studies also demonstrated the correlation between asthma severity and SRBD [27]. In children, based on the result of a recent systematic review, Sánchez et al concluded that children with asthma were more likely to develop habitual snoring and SRBD, and children with SRBD were more likely to develop asthma. Moreover, the authors found out that asthma was associated with more severe OSA, and the presence of SRDB was associated with severe asthma [28]. Similarly, an estimated prevalence of up to 33% sleep-disordered breathing was suggested among 194 inner city 4- 10 year old children who were enrolled in a school-based asthma intervention program, particularly among those with persistent asthma, suggesting that asthma severity is associated with dose-dependent increases in the prevalence of SRBD [29]. However, the bidirectional correlation between asthma and SRDB is still controversial.
According to the GINA guidelines, lung function is important for determining the level of asthma control [30]; however, the relationship between lung function and SRBD is unclear. In a previous study, Sheen et al. enrolled 220 children with asthma and revealed that FEV1/forced vital capacity is associated with the PSQ score [31]. However, another research showed that there is no difference between the lung functions of patients with a high risk of SRBD and those of patients with a low risk of SRBD [11]. In the present study, we did not find a relationship between lung function and SBD.
Although we anticipated increases in the frequency of SRBD, the extremely high prevalence of SRBD in this cohort was somewhat unexpected, and could be due not only to the underlying asthmatic condition.
Upper airway inflammatory processes may play a role in lower airway inflammation and asthma, and conversely lower airway inflammatory disease may promote adenotonsillar proliferation, and therefore increase the propensity for SRBD [21,32]. However, Upper airway inflammatory processes may play a role in lower airway inflammation and asthma, and conversely lower airway inflammatory disease may promote adenotonsillar proliferation, and therefore increase the propensity for SRBD [21,32]. However, study was not designed to assess such interactions, and such assessment will require a larger prospective cohort and different study design [33-35].
This study, though, was subjected to some limitations. Firstly, as an observational, cross-sectional, pilot study with a small sample size, it cannot determine any causal relationship. Secondly, the risk of SRBD was assessed by a non-validated Vietnamese PSQ questionnaire. Since children with asthma who were classified as low risk of developing SRBD according to PSQ were found to have SRBD via polysomnography [36]. Another missing aspect in this work is the consideration of gastroesophageal reflux, which is well known as a risk factor for both diseases in children, and gastroesophageal reflux was also associated with SRDS because of trigger bronchoconstriction through altered parasympathetic tone or other mechanisms [10,37-42].
Asthma is a common disease and affects patients of all ages and both genders [43,44]. Once the diagnosis of asthma is confirmed and adherence to the treatment plan with appropriate inhaler technique is assessed, it is important to thoroughly examine other factors or comorbidities that can contribute to poor asthma control. SRBD is one contributing factor, particularly in patients with nocturnal symptoms [45,46].
CONCLUSION
In summary, we present preliminary observations that the prevalence of SRBD is exceedingly high in poorly controlled asthmatic children. Children with uncontrolled asthma are more likely to have poor sleep quality and sleep-associated problems than children with well-controlled asthma.
Poorly controlled asthma increase the risk of having SRBD. The coexistence of SRBD and asthma may have a cumulative effect, in terms of morbidity, so both disturbances must be recognized and treated promptly.
CONFLICT OF INTERESTS
Non.
REFERENCES
| 1. Global Initiative for Asthma. Global strategy for asthma management and prevention (updated 2021): Definition, description and diagnosis of asthma. |
| 2. Tham EH, Lee AJ, Bever HV. Aeroallergen sensitization and allergic disease phenotypes in Asia. Asian Pacific journal of allergy and immunology/launched by the Allergy and Immunology Society of Thailand. 2016;34(3):181-9. |
| 3. Sanchez T, Rojas C, Casals M et al. Prevalence and risk factors for sleep-disordered breathing in chilean school children. Revista Chilena de Pediatria. 2018; 89: 718- 725. |
| 4. Li L, Xu Z, Jin X et al. Sleep-disordered breathing and asthma: evidence from a large multicentric epidemiological study in China. Respiratory research. 2015;16:56. |
| 5. Zandieh SO, Cespedes A, Ciarleglio A et al. Asthma and subjective sleep disordered breathing in a large cohort of urban adolescents. Journal of Asthma. 2017; 54 (1); 62-68. |
| 6. Brockmann PE, Bertrand P, Castro-Rodriguez JA. Influence of asthma on sleep disordered breathing in children: a systemic review. Sleep Medicine Reviews. 2014; 18 (5); 393- 397. |
| 7. Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. Journal of investigative medicine: the official publication of the American Federation for Clinical Research. 2014;62(4):665-75. |
| 8. Ginis T, Akcan FA, Capanoglu M et al. The frequency of sleep- disordered breathing in children with asthma and its effects on asthma control. J Asthma. 2017; 54: 403-410. |
| 9. Banasiak NC. Understanding the relationship between asthma and sleep in the pediatric population. Journal of Pediatric Health Care. 2016; 30(6); 546- 550. |
| 10. Ross KR, Storfer-Isser A, Hart MA et al. Sleep- disordered breathing is associated with asthma severity in children. The Journal of Pediatrics. 2012; 160 (5); 736- 742. |
| 11. Perikleous E, Steiropoulos P, Nena E et al. Association of asthma and allergic rhinitis with sleep- disordered breathing in childhood. Frontiers in Pediatrics. 2018; 6: 250. |
| 12. Chervin RD, Hedger K, Dillon JE et al. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000; 1(1):21-32. |
| 13. Kaditis AG, Alvarez MLA, Boudewyns A et al. Obstructive sleep disordered breathing in 2 to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2016, 47, 69–94. |
| 14. Burghard M, Brozek-Madry E, Krzeski A. Sleep disordered breathing in children - Diagnostic questionnaires, comparative analysis. Int. J. Pediatr. Otorhinolaryngol. 2019; 120; 108–111. |
| 15. Thomas M, Kay S, Pike J et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009; 18(1):41- 49. |
| 16. Liu AH, Zeiger R, Sorkness C et al. Development and cross- sectional validation of the childhood asthma control test. J Allergy Clin Immun. 2007; 119: 817-825. |
| 17. Duong-Quy S. Clinical Utility Of The Exhaled Nitric Oxide (NO) Measurement With Portable Devices In The Management Of Allergic Airway Inflammation And Asthma. J Asthma Allergy. 2019 Oct 7;12:331-341. |
| 18. Shypailo RJ. Age-based Pediatric Growth Reference Charts. Retrieved 12/4/2021 from the Baylor College of Medicine, Children's Nutrition Research Center. |
| 19. Nguyen VN, Chavannes N, Le LT et al. The Asthma Control Test (ACT) as an alternative tool to Global Initiative for Asthma (GINA) guideline criteria for assessing asthma control in Vietnamese outpatients. Prim Care Respir J. 2012;21(1):85-89. |
| 20. Guo Y, Zhang X, Liu F et al. Relationship between Poorly Controlled Asthma and Sleep-Related Breathing Disorders in Children with Asthma: A Two-Center Study. Can Respir J. 2021 Jan 28;2021:8850382. |
| 21. Léger D, Annesi-Maesano I, Carat F, et al. Allergic rhinitis and its consequences on quality of sleep: an unexplored area. Arch Intern Med. 2006;166(16):1744–1748. |
| 22. Suratt PM, Turner BL, Wilhoit SC. Effect of intranasalobstruction on breathing during sleep. Chest. 1986;90(3):324–329. |
| 23. Duong-Quy S. Obstructive Sleep Apnea (OSA) in children: Fact and Challenge. J Func Vent Pulm 2018;9 (27), 1-2 |
| 24. Ho-Viet TD, Duong-Quy S, Timothy J C. Allergic rhinitis, upper airway and quality of sleep. J Func Vent Pulm 2017;8 (24), 3-9. |
| 25. Duong-Quy S. Allergic rhinitis and asthma: one disease of two organs. J Func Vent Pulm 2017;8 (24), 1-2. |
| 26. S Duong-Quy, T Nguyen-Thi-Dieu, K Tran-Quang, T Tang-Thi-Thao, Toi Nguyen-Van, Thu Vo-Pham-Minh, Quan Vu-Tran-Thien, Khue Bui-Diem, Vinh Nguyen-Nhu, Lam Hoang-Thi, Timothy Craig. Study of Nasal Fractional Exhaled Nitric Oxide (FENO) in Children with Allergic Rhinitis. Sinusitis 2021; 5 (2), 123-131. |
| 27. Nguyen-Hoang Y, Nguyen-Thi-Dieu T, Duong-Quy S. Study of the clinical and functional characteristics of asthmatic children with obstructive sleep apnea. J Asthma Allergy 2017, 10:285-292. |
| 28. Sánchez T, Castro-Rodríguez JA, Brockmann PE. Sleep-disordered breathing in children with asthma: a systematic review on the impact of treatment. J Asthma Allergy. 2016;9:83–91. |
| 29. Fagnano M, van Wijngaarden E, Connolly HV et al. Sleep-disordered breathing and behaviors of inner-city children with asthma. Pediatrics. 2009; 124(1): 218–225. |
| 30. Dang-The P, Tran-Van T, Duong-Quy S. Study of forced expiratory flow of 25%-75% values (FEF25-75) in the control of asthma according to GINA. J Func Vent Pulm 2020; 11 (33), 42-46. |
| 31. Sheen YH, Choi SH, Jang SJ et al. Poor sleep quality has an adverse effect in childhood asthma control and lung functions measures. Pediatrics International. 2017; 59 (8): 917- 922. |
| 32. Dayyat E, Kheirandish-Gozal L, Sans Capdevila O et al. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009 Jul; 136(1):137–44. |
FIGURE - TABLES
REFERENCES
| 1. Global Initiative for Asthma. Global strategy for asthma management and prevention (updated 2021): Definition, description and diagnosis of asthma. |
| 2. Tham EH, Lee AJ, Bever HV. Aeroallergen sensitization and allergic disease phenotypes in Asia. Asian Pacific journal of allergy and immunology/launched by the Allergy and Immunology Society of Thailand. 2016;34(3):181-9. |
| 3. Sanchez T, Rojas C, Casals M et al. Prevalence and risk factors for sleep-disordered breathing in chilean school children. Revista Chilena de Pediatria. 2018; 89: 718- 725. |
| 4. Li L, Xu Z, Jin X et al. Sleep-disordered breathing and asthma: evidence from a large multicentric epidemiological study in China. Respiratory research. 2015;16:56. |
| 5. Zandieh SO, Cespedes A, Ciarleglio A et al. Asthma and subjective sleep disordered breathing in a large cohort of urban adolescents. Journal of Asthma. 2017; 54 (1); 62-68. |
| 6. Brockmann PE, Bertrand P, Castro-Rodriguez JA. Influence of asthma on sleep disordered breathing in children: a systemic review. Sleep Medicine Reviews. 2014; 18 (5); 393- 397. |
| 7. Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. Journal of investigative medicine: the official publication of the American Federation for Clinical Research. 2014;62(4):665-75. |
| 8. Ginis T, Akcan FA, Capanoglu M et al. The frequency of sleep- disordered breathing in children with asthma and its effects on asthma control. J Asthma. 2017; 54: 403-410. |
| 9. Banasiak NC. Understanding the relationship between asthma and sleep in the pediatric population. Journal of Pediatric Health Care. 2016; 30(6); 546- 550. |
| 10. Ross KR, Storfer-Isser A, Hart MA et al. Sleep- disordered breathing is associated with asthma severity in children. The Journal of Pediatrics. 2012; 160 (5); 736- 742. |
| 11. Perikleous E, Steiropoulos P, Nena E et al. Association of asthma and allergic rhinitis with sleep- disordered breathing in childhood. Frontiers in Pediatrics. 2018; 6: 250. |
| 12. Chervin RD, Hedger K, Dillon JE et al. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000; 1(1):21-32. |
| 13. Kaditis AG, Alvarez MLA, Boudewyns A et al. Obstructive sleep disordered breathing in 2 to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2016, 47, 69–94. |
| 14. Burghard M, Brozek-Madry E, Krzeski A. Sleep disordered breathing in children - Diagnostic questionnaires, comparative analysis. Int. J. Pediatr. Otorhinolaryngol. 2019; 120; 108–111. |
| 15. Thomas M, Kay S, Pike J et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009; 18(1):41- 49. |
| 16. Liu AH, Zeiger R, Sorkness C et al. Development and cross- sectional validation of the childhood asthma control test. J Allergy Clin Immun. 2007; 119: 817-825. |
| 17. Duong-Quy S. Clinical Utility Of The Exhaled Nitric Oxide (NO) Measurement With Portable Devices In The Management Of Allergic Airway Inflammation And Asthma. J Asthma Allergy. 2019 Oct 7;12:331-341. |
| 18. Shypailo RJ. Age-based Pediatric Growth Reference Charts. Retrieved 12/4/2021 from the Baylor College of Medicine, Children's Nutrition Research Center. |
| 19. Nguyen VN, Chavannes N, Le LT et al. The Asthma Control Test (ACT) as an alternative tool to Global Initiative for Asthma (GINA) guideline criteria for assessing asthma control in Vietnamese outpatients. Prim Care Respir J. 2012;21(1):85-89. |
| 20. Guo Y, Zhang X, Liu F et al. Relationship between Poorly Controlled Asthma and Sleep-Related Breathing Disorders in Children with Asthma: A Two-Center Study. Can Respir J. 2021 Jan 28;2021:8850382. |
| 21. Léger D, Annesi-Maesano I, Carat F, et al. Allergic rhinitis and its consequences on quality of sleep: an unexplored area. Arch Intern Med. 2006;166(16):1744–1748. |
| 22. Suratt PM, Turner BL, Wilhoit SC. Effect of intranasalobstruction on breathing during sleep. Chest. 1986;90(3):324–329. |
| 23. Duong-Quy S. Obstructive Sleep Apnea (OSA) in children: Fact and Challenge. J Func Vent Pulm 2018;9 (27), 1-2 |
| 24. Ho-Viet TD, Duong-Quy S, Timothy J C. Allergic rhinitis, upper airway and quality of sleep. J Func Vent Pulm 2017;8 (24), 3-9. |
| 25. Duong-Quy S. Allergic rhinitis and asthma: one disease of two organs. J Func Vent Pulm 2017;8 (24), 1-2. |
| 26. S Duong-Quy, T Nguyen-Thi-Dieu, K Tran-Quang, T Tang-Thi-Thao, Toi Nguyen-Van, Thu Vo-Pham-Minh, Quan Vu-Tran-Thien, Khue Bui-Diem, Vinh Nguyen-Nhu, Lam Hoang-Thi, Timothy Craig. Study of Nasal Fractional Exhaled Nitric Oxide (FENO) in Children with Allergic Rhinitis. Sinusitis 2021; 5 (2), 123-131. |
| 27. Nguyen-Hoang Y, Nguyen-Thi-Dieu T, Duong-Quy S. Study of the clinical and functional characteristics of asthmatic children with obstructive sleep apnea. J Asthma Allergy 2017, 10:285-292. |
| 28. Sánchez T, Castro-Rodríguez JA, Brockmann PE. Sleep-disordered breathing in children with asthma: a systematic review on the impact of treatment. J Asthma Allergy. 2016;9:83–91. |
| 29. Fagnano M, van Wijngaarden E, Connolly HV et al. Sleep-disordered breathing and behaviors of inner-city children with asthma. Pediatrics. 2009; 124(1): 218–225. |
| 30. Dang-The P, Tran-Van T, Duong-Quy S. Study of forced expiratory flow of 25%-75% values (FEF25-75) in the control of asthma according to GINA. J Func Vent Pulm 2020; 11 (33), 42-46. |
| 31. Sheen YH, Choi SH, Jang SJ et al. Poor sleep quality has an adverse effect in childhood asthma control and lung functions measures. Pediatrics International. 2017; 59 (8): 917- 922. |
| 32. Dayyat E, Kheirandish-Gozal L, Sans Capdevila O et al. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009 Jul; 136(1):137–44. |
ARTICLE INFO DOI: 10.12699/jfvpulm.12.38.2021.8
Conflict of Interest
Non
Date of manuscript receiving
25/02/2021
Date of publication after correction
25/08/2021
Article citation
Nguyen-Ngoc-Q, Luong-Thi L, Le-Quynh C, D. Tran M, Nguyen Thi Thanh M, Nguyen Thi Dieu T, Duong-Quy S. Association of sleep-related breathing disorders with asthma control in children. J Func Vent Pulm 2021;38(12):8-14