
 English
English
 French
French
Obstructive sleep apnea in children with attention deficit hyperactivity disorder: A literature review
Apnée obstructive du sommeil chez les enfants atteints de trouble du déficit de l'attention avec hyperactivité: Une revue de la ittérature
Mai Nguyen-Thi-Phuong1, Mai Nguyen-Thi-Thanh1, Sy Duong-Quy2,3
1: Hanoi Medical University, Hanoi, Vietnam
2: Sleep Lab Center of Vietnam Society of Sleep Medicine. Dalat, Vietnam
3: Bio-Medical Research Center. Lam Dong Medical College. Dalat, Vietnam
Corresponding author: Sy Duong-Quy. Sleep Lab Center of Vietnam Society of Sleep Medicine. Dalat, Vietnam
E-mail: sduongquy.jfvp@gmail.com
ABSTRACT
This review explores the relationship between Obstructive Sleep Apnea (OSA) and Attention Deficit Hyperactivity Disorder (ADHD) in children, focusing on comorbidity rates, pathophysiology, and treatment strategies.
Studies show that OSA is more prevalent in children with ADHD, and sleep fragmentation along with intermittent hypoxia caused by OSA can exacerbate ADHD symptoms such as inattention, hyperactivity, and impulsivity. Effective treatments for OSA, including adenotonsillectomy and Continuous Positive Airway Pressure (CPAP), have been shown to improve ADHD symptoms and cognitive function. Given the complex interaction between these conditions, early diagnosis and intervention are crucial to improving the quality of life for affected children.
The strong relationship between OSA and ADHD in children has been demonstrated through numerous studies. OSA can exacerbate ADHD symptoms through mechanisms of hypoxia and sleep fragmentation, impacting cognitive and behavioral functions. Early diagnosis and appropriate treatment of OSA can significantly improve the quality of life for children with ADHD, with adenotonsillectomy and CPAP being effective treatment options. Further research is necessary to optimize treatment approaches and to better understand the mechanisms linking OSA and ADHD.
KEYWORDS: ADHD; Obstructive Sleep Apnea; Children.
RÉSUMÉ
Cette revue explore la relation entre l'apnée obstructive du sommeil (AOS) et le trouble du déficit de l'attention avec hyperactivité (TDAH) chez les enfants, en concentrant sur les taux de comorbidité, la physiopathologie et les stratégies de traitement.
Des études montrent que l'AOS est plus fréquente chez les enfants atteints de TDAH, et la fragmentation du sommeil ainsi que l'hypoxie intermittente causée par l'AOS peuvent aggraver les symptômes du TDAH tels que l'inattention, l'hyperactivité et l'impulsivité. Des traitements efficaces pour l'AOS, y compris l'adénoïdectomie et la pression positive continue des voies respiratoires (CPAP), ont montré qu'ils améliorent les symptômes du TDAH et la fonction cognitive. Étant donné l'interaction complexe entre ces conditions, un diagnostic précoce et une intervention sont cruciaux pour améliorer la qualité de vie des enfants concernés.
La relation étroite entre l'apnée obstructive du sommeil (AOS) et le trouble du déficit de l'attention avec ou sans hyperactivité (TDAH) chez les enfants a été démontrée par de nombreuses études. L'AOS peut exacerber les symptômes du TDAH par des mécanismes d'hypoxie et de fragmentation du sommeil, affectant ainsi les fonctions cognitives et comportementales. Un diagnostic précoce et un traitement approprié de l'AOS peuvent considérablement améliorer la qualité de vie des enfants atteints de TDAH, avec l'adéno-amygdalectomie et la thérapie CPAP comme options de traitement efficaces. Des recherches supplémentaires sont nécessaires pour optimiser les approches thérapeutiques et mieux comprendre les mécanismes reliant l'AOS et le TDAH.
Des recherches supplémentaires sont nécessaires pour optimiser les approches de traitement et mieux comprendre les mécanismes liant l'AOS et le TDAH
MOTS CLÉS: TDAH; Apnée obstructive du sommeil; Enfants.
INTRODUCTION
Attention Deficit Hyperactivity Disorder (ADHD) is one of the most common neurodevelopmental disorders in children, characterized by two groups of symptoms: inattention and/or hyperactivity-impulsivity [1]. This disorder often persists into adulthood and is a risk factor for several mental health conditions, such as conduct disorder, oppositional defiant disorder, mood disorders, substance abuse, and criminal behavior…[2] The estimated global prevalence of this disorder in children is around 5 - 7%, [3] and it is showing a rising trend [4,5,6].
Meanwhile, treating ADHD often faces significant challenges due to comorbid disorders, behavioral management issues, and financial burdens.7 Clinical studies show that children with ADHD often have comorbid mental health issues, which complicate treatment and further reduce the already low quality of life for both the children and their families. Among the most common comorbidities are sleep disorders [8,9], particularly Obstructive Sleep Apnea (OSA), a sleep-related breathing disorder closely associated with ADHD [10]. OSA has garnered significant attention from researchers and clinicians alike. Children with OSA frequently exhibit sleep-related symptoms such as loud snoring, episodes of apnea, and restless sleep, as well as daytime symptoms like excessive daytime sleepiness and behavioral abnormalities similar to ADHD [11].
The relationship between OSA and ADHD is not merely a clinical overlap but involves complex interactions [12]. It is hypothesized that the sleep fragmentation and intermittent hypoxemia characteristic of OSA lead to metabolic changes in specific brain regions, potentially impairing executive function and exacerbating ADHD symptoms. Additionally, improper treatment or delayed diagnosis of OSA may reduce the effectiveness of ADHD therapies [12]. Therefore, understanding the mechanisms, diagnosis, and treatment of OSA in children with ADHD is crucial to improving their quality of life. This paper aims to provide a comprehensive overview of the epidemiology, pathophysiology, diagnostic methods, and treatment of OSA in children with ADHD, offering a thorough understanding of the connection between these two disorders.
Prevalence of OSA in children with ADHD
OSA results from anatomical or functional narrowing of the upper airway, which includes upper airway obstruction, reduced muscle tone in the pharyngeal region, and/or impaired breathing during obstruction [13]. In children, the prevalence of OSA is approximately 1-5%, with the highest rates occurring in those aged 2-8 years, as this age group has the highest incidence of tonsillar hypertrophy [13].
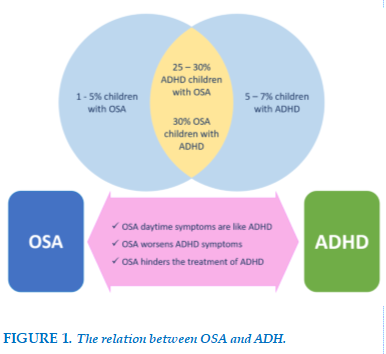
The prevalence rate appears to vary depending on the assessment tools and study populations. Approximately 4-11% of children are diagnosed with OSA based on clinical symptoms reported by parents [11], while polysomnography data shows a prevalence of 1-7%, with 2-3% of cases classified as severe OSA [13]. However, this rate is significantly higher in children with ADHD. A meta-analysis by Cortese et al. provided objective evidence showing that the prevalence of OSA in children with ADHD is around 25-30% [14]. A study using polysomnography in children with ADHD revealed that up to 50% of them showed signs of sleep-related breathing disorders, which was significantly higher compared to the control group [15].
Interestingly, the prevalence of ADHD also increases in children with OSA. Specifically, Johnson et al. observed in a study of 1,114 children aged 13-16 that sleep-disordered breathing (SDB) doubled the risk of developing ADHD [16]. A study on children with OSA reported that 30% met the diagnostic criteria for ADHD [17]. This provides compelling evidence that the prevalence of OSA is higher in children with ADHD, and vice versa, highlighting the need for careful evaluation of both conditions.
Additionally, the strong link between OSA and ADHD is further supported by clinical studies showing an improvement in ADHD symptoms after adenotonsillectomy [18,19], One study involving 105 children aged 5-12 years found improvements in hyperactivity and attention deficits one year after the surgery [18].
Another study on children with ADHD and mild OSA, diagnosed through polysomnography, allowed parents to choose between treatment with methylphenidate or adenotonsillectomy for OSA without using methylphenidate. Results showed that the surgical group had superior outcomes compared to the group treated with methylphenidate [19]. FIGURE 1
Pathophysiology
Sleep fragmentation and intermittent hypoxia, which are characteristic of OSA, can disrupt the body's restorative processes during sleep, triggering a series of biochemical and cellular disturbances.
These lead to a breakdown of homeostasis and alter the viability of neurons in various brain regions, particularly in the prefrontal cortex.
Executive dysfunction in the prefrontal cortex can affect cognitive functions, leading to behavioral disorders, reduced cognitive flexibility, and impaired emotional regulation in response to environmental stimuli.
It also diminishes analytical and memory abilities, resulting in behaviors that resemble ADHD symptoms during the day [12].
Obstructive Sleep Apnea (OSA) leads to repetitive episodes of intermittent hypoxia (IH), which significantly contributes to central nervous system (CNS) damage.
IH triggers oxidative stress, inflammation, and neuronal apoptosis through several mechanisms [20].
Oxidative Stress
IH increases the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), leading to oxidative damage to neurons, particularly in the hippocampus and prefrontal cortex, regions vital for memory and executive function.
Inflammatory Response
Microglia, the resident immune cells in the CNS, are activated by IH, producing pro-inflammatory cytokines such as TNF-α and IL-1β. This leads to a chronic inflammatory state that exacerbates neuronal damage.
Excitotoxicity
IH elevates the levels of excitatory neurotransmitters like glutamate, which overstimulates neurons, resulting in excitotoxicity and further neuronal death.
Neurodegeneration
Over time, the cumulative effect of oxidative stress, inflammation, and excitotoxicity leads to the loss of neurons and structural damage in critical areas of the brain, contributing to cognitive impairments seen in OSA patients.
Reactive microgliosis releases pro-inflammatory factors in a self-reinforcing, self-sustaining, and self-amplifying manner.
Over time, this slow, chronic inflammatory process in the CNS progressively destroys enough neurons to manifest as clinical symptoms of CNS diseases.
Numerous studies have provided compelling evidence of a range of behavioral and cognitive disorders in children with OSA (diagnosed via polysomnography), including reduced attention, memory impairments, executive dysfunction, mood disorders, behavioral problems, and learning difficulties [21-23].
These studies also report that children with OSA have a 2.4 times higher risk of hyperactivity, a 4 times higher risk of inattention, and a 9.6 times higher risk of peer relationship problems compared to controls [23].
Additionally, studies examining behavioral and neurocognitive changes in children after OSA treatment (typically through tonsillectomy) have documented significant improvements in daytime sleepiness, behavioral problems, and academic performance. Specifically, 50% of children who met the DSM-IV criteria for ADHD showed marked improvements one year after tonsillectomy [24,25].
Clinical Characteristics
The clinical manifestations of OSA are highly variable and non-specific, often observed and reported by parents or caregivers [11].
OSA symptoms can vary by age. In infants, particularly those with a history of prematurity, symptoms may include "noisy" breathing, night sweats, restless sleep, frequent nighttime awakenings, excessive crying during the night, poor feeding, and slow growth. In school-aged children, OSA tends to resemble adult OSA more closely, with risk factors such as obesity and clinical manifestations like snoring, sleep apnea, and excessive daytime sleepiness.
The nighttime sleep-related symptoms of OSA
Snoring and sleep apnea episodes
Children with OSA often snore loudly and continuously. Parents frequently describe episodes of chest retraction and labored breathing, with increased respiratory effort.
When part or all of the upper airway is obstructed, the downward movement of the diaphragm during the effort to inhale causes the abdomen to expand.
However, the sudden increase in negative pressure in the chest leads to a paradoxical movement of the chest wall. These episodes of apnea or hypopnea may end with gasping for air, a change in position, or frequent awakenings.
Restlessness at night
Children may exhibit restless sleep, frequently change their sleeping positions,
and tilt their heads back to keep their airways open. Obese children with severe obstructive sleep apnea may prefer to sleep sitting upright or propped up on pillows. Other symptoms: Additional signs include night sweats, bedwetting, dry mouth, drooling, and teeth grinding during sleep. Studies have shown that the most common OSA symptoms are snoring, mouth breathing, labored breathing, and observed apnea episodes at night [26].
Daytime symptoms
The most common complaints from parents include mouth breathing, frequent respiratory infections, as well as issues with hearing and speech. Symptoms like nausea, vomiting, or difficulty swallowing are also commonly observed.
Daytime sleepiness in children has been shown to correlate with the severity of OSA and an increased body mass index [27,28]. However, unlike in adults, daytime sleepiness is less common in children with OSA, particularly those with ADHD.
Behavioral abnormalities are also common and are believed to result from repeated sleep disruptions and intermittent hypoxia caused by apnea or hypopnea, which affect executive functions such as memory, behavior, cognition, and emotional regulation due to sleep disturbances. The behavioral manifestations in children with OSA can closely resemble those of children with ADHD.
Physical symptoms
A thorough physical examination is essential for evaluating patients with OSA. Key issues to screen for include asthma, allergic rhinitis, tonsillar hypertrophy, adenoid hypertrophy, upper airway abnormalities, and neuromuscular disorders.
Potential consequences of OSA
Learning and behavior: Children with sleep apnea often exhibit disruptive behavior, irritability, and reduced concentration.
Social: A child's loud snoring may disturb others who share the same sleeping space.
Physical development: OSA can lead to insufficient production of growth hormone, potentially affecting the child's physical development.
Nocturnal enuresis: OSA may cause an increase in nighttime urine production, which can lead to bedwetting.
Obesity: OSA can contribute to obesity by increasing insulin resistance and causing daytime fatigue, which reduces the child's ability to engage in physical activity.
Cardiovascular: OSA is often associated with an increased risk of hypertension and other cardiovascular issues.
Diagnosis of OSA in Children with ADHD
Pediatric Sleep Questionnaire
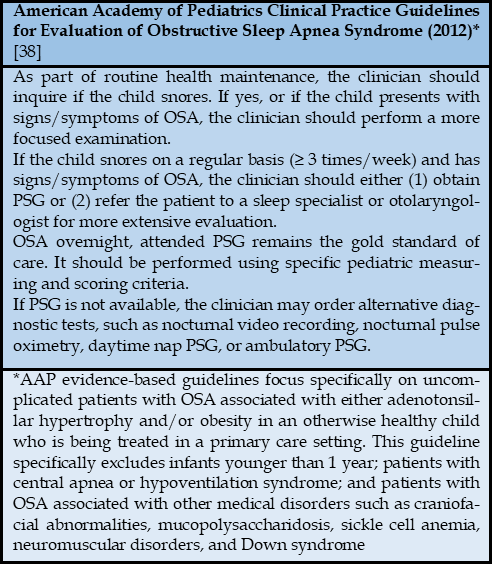
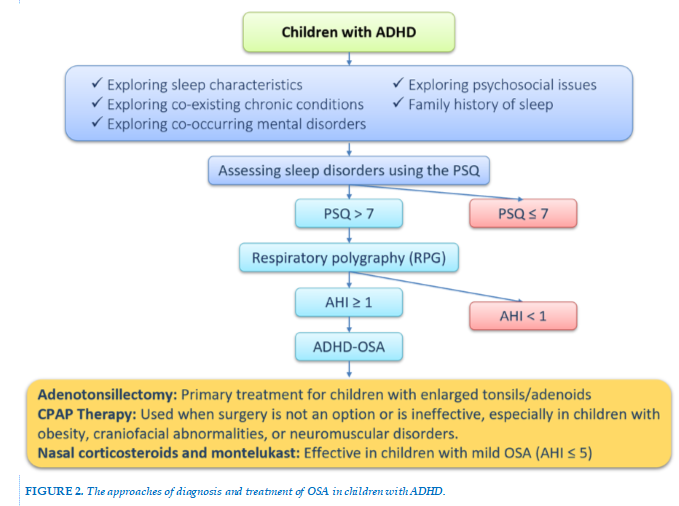
All children exhibiting ADHD symptoms should be screened for OSA due to the complex relationship between these two disorders. In clinical practice, the most commonly used screening tool for OSA is the Pediatric Sleep Questionnaire (PSQ) [29]. This questionnaire, developed by Chervin and colleagues in the early 2000s, is designed for children aged 2 to 18 years and is completed by the caregiver [29]. The PSQ consists of 22 questions divided into three main categories: 7 questions on sleep disturbances, 9 questions on snoring, and 6 questions on hyperactivity-inattention, based on the ADHD criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Each item is answered with a simple "yes" or "no," scoring 1 or 0 points, respectively. If more than 7 responses are "yes," obstructive sleep apnea in children should be considered. The sensitivity of the scale ranges from 0.81 to 0.85, with a specificity of 0.87, and the Cronbach's alpha coefficient ranges from 0.66 to 0.89 [29]. The European Respiratory Society recognizes the PSQ as an effective tool for screening OSA, identifying its neurodevelopmental impact on children, and assessing treatment response to OSA [30]. Currently, the PSQ is widely used as a screening tool for OSA in children worldwide, including in Vietnam [29].
Polysomnography
Polysomnography (PSG) is the gold standard for diagnosing OSA in children because no single symptom or physical finding reliably distinguishes primary snoring from other sleep-related breathing disorders [31,32]. This means that snoring and symptoms of sleep-related breathing disorders can occur even without recorded respiratory abnormalities. Moreover, the reliability of reported symptoms is quite low, particularly in older children who sleep alone and are not observed by caregivers. Airway obstruction tends to worsen during REM sleep, which typically occurs more frequently in the last third of the night, when caregivers are likely to be in a deep sleep and may miss the child’s breathing abnormalities [33]. According to the American Academy of Sleep Medicine (AASM), there are four types of PSG, based on the method of channel recording and the presence of a sleep technician during the recording process to monitor the procedure ("attended" or "unattended") [34].
Type I PSG is conducted in a sleep laboratory with the presence of a sleep technician, recording a minimum of seven channels, including electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), electrocardiogram (ECG), airflow through the nose and mouth, oxygen saturation (SpO2), and snoring.
Type II PSG is essentially Type I PSG recorded without the supervision of a sleep technician, and can be performed either in or outside of the sleep laboratory.
Type III PSG, also known as respiratory polygraphy, is primarily developed for diagnosing and monitoring OSA. This simplified device includes four channels: nasal and oral airflow, 1 or 2 respiratory effort channels, oxygen saturation, and ECG or heart rate, and is performed at home or outside the sleep lab without supervision [35].
Type IV PSG records two to three channels, usually airflow, SpO2, and heart rate, and is typically conducted at home without supervision.
PSG plays a crucial role in the diagnosis and treatment of various sleep disorders in children. Specific indications for PSG in children include the following [31,32].
Diagnosis of sleep-related breathing disorders such as OSA, central sleep apnea (CSA), or sleep-related hypoventilation disorders.
Preoperative evaluation for upper airway surgery, especially in children who snore, show signs and symptoms of OSA, or have other high-risk features (e.g., obesity, Down syndrome, craniofacial abnormalities, neuromuscular disorders, sickle cell disease).
A CPAP titration study, which is an overnight sleep study used to properly set continuous positive airway pressure (CPAP) therapy, is conducted for sleep-related breathing disorders.
Evaluation of treatment efficacy in patients with sleep-related breathing disorders.
Assessment and diagnosis of suspected narcolepsy or excessive daytime sleepiness.
Evaluation of sudden nocturnal events using EEG and video-PSG in cases of suspected sleep-related seizures or potentially injurious behaviors.
Assisting in the diagnosis of Restless Legs Syndrome (RLS) and Periodic Limb Movement Disorder (PLMD).
In the appropriate clinical context, PSG often provides a definitive diagnosis for a range of sleep-related breathing disorders, such as OSA, CSA, and narcolepsy type 1 and type 2 (in combination with the Multiple Sleep Latency Test - MSLT), as well as Periodic Limb Movement Disorder. However, clinical evaluation should be combined with PSG for an accurate diagnosis.
Other Diagnostic Tests
Endoscopy otolaryngology is used to assess adenoid and tonsillar hypertrophy. Observing posterior pharyngeal wall edema may suggest signs of gastroesophageal reflux, a contributing factor to airway narrowing.
Drug-induced sleep endoscopy is a relatively new and effective diagnostic tool for identifying sites of obstruction under conditions that closely mimic normal sleep. It can be particularly useful for evaluating lingual tonsil hypertrophy and underlying laryngomalacia. Other diagnostic tests: Chest X-rays may reveal right ventricular hypertrophy in severe OSA, and ECG may also show evidence of right ventricular hypertrophy. Additional tools, such as cranial X-rays, CT scans, or MRI, can be helpful in evaluating upper airway structures, especially in children with craniofacial abnormalities. Most blood tests are unnecessary in OSA, although severe cases may show polycythemia on a complete blood count and/or metabolic alkalosis related to chronic hypoventilation [36].
Diagnostic Criteria for OSA in Children
According to ICSD-3, the diagnostic criteria for OSA in children require the fulfillment of both criteria A and B [37]. (A) The presence of one or more of the following: (1) snoring, (2) labored breathing, abnormal breathing patterns, or obstructed breathing during sleep, (3) daytime sleepiness, hyperactivity, behavioral problems, or learning difficulties; (B) More than 1 obstructive respiratory event per hour of sleep, or evidence of obstructive hypoventilation (PaCO2 > 50 mmHg) for more than 25% of sleep time, accompanied by snoring, a flattened airway pressure waveform, or abnormal chest movements. The severity of OSA is determined by the Apnea-Hypopnea Index (AHI), where ≥1 event per hour meets the OSA criteria. An AHI of 1–5 events per hour is considered mild, 6–10 events per hour is moderate, and >10 events per hour is classified as severe [37].
Management of OSA in Children with ADHD
There are various treatment options for OSA in children, including adenotonsillectomy, turbinate surgery, maxillofacial surgery, and palatal surgery, which are indicated based on the cause of airway obstruction. Other treatments include continuous positive airway pressure (CPAP), positional therapy, and weight management. These treatment methods are also applied in children with ADHD who have OSA [36]. The most common cause of OSA in children is adenotonsillar hypertrophy. Therefore, adenotonsillectomy is typically recommended as the first-line therapy for OSA in children with enlarged tonsils/adenoids [39].
Studies investigating the effects of this surgery as a treatment for OSA have shown cure rates of 85 - 95% [40]. Systematic reviews and meta-analyses have also demonstrated significant improvements in OSA severity following surgery [41]. Additionally, numerous prospective cohort studies have shown the benefits of adenotonsillectomy in addressing the adverse neurobehavioral consequences and quality of life in children with OSA. These studies consistently report improvements in outcome measures such as quality of life, behavioral issues including hyperactivity/impulsivity and aggression, as well as neurocognitive skills such as memory, attention, and academic performance [42,43].
A study involving 254 children reported the impact of adenotonsillectomy on IQ, with mixed results, indicating that younger children seemed to benefit more [44]. Studies on the outcomes of adenotonsillectomy for OSA in children with ADHD show that at least 50% of children with ADHD no longer meet the diagnostic criteria for ADHD after surgery [42,45]. Several prospective cohort studies from Iran [46,47] and Turkey [45,48] have compared ADHD symptoms using standardized tools before and after adenotonsillectomy, all showing improvements in ADHD symptoms 3-6 months post-surgery [47].
Recurrent upper airway inflammation is common in children with OSA [49], and increased expression of glucocorticoid receptors alpha and beta, as well as leukotriene C4 synthase, has been observed in hypertrophic lymphoid tissues of the tonsils/adenoids in children with OSA [49,50]. As a result, corticosteroids and oral leukotriene receptor antagonists have been tested as potential treatments for OSA. Nasal corticosteroids and montelukast have shown favorable outcomes in trials involving children with OSA [51,52]. Treatment duration in these studies typically ranged from 6 weeks for nasal corticosteroids to 12-16 weeks for montelukast. Most trials focused on children with mild OSA (AHI ≤ 5) and demonstrated significant improvements in AHI. However, these studies did not measure the impact of treatment on outcomes such as quality of life, cognitive and behavioral effects, and none of the medical treatment studies specifically targeted children with ADHD.
Continuous positive airway pressure (CPAP) involves delivering a continuous flow of air through a mask, acting as a splint for the upper airway to prevent airway collapse. It is used as a treatment option in children when adenotonsillectomy or other interventions do not result in adequate clinical improvement or when surgery is not indicated. Obese children, or those with craniofacial abnormalities or neuromuscular disorders, are the most common candidates for CPAP treatment [53]. Although no studies specifically report on CPAP use in children with ADHD and OSA, some studies have examined the benefits of CPAP on daytime functioning in children aged 2 - 16. These studies evaluated issues such as inattention, daytime sleepiness, behavior, and quality of life based on reports from both children and caregivers after 3 months of CPAP treatment [53,54].While the subjects were not diagnosed with ADHD, results indicated statistically significant improvements in hyperactivity and attention deficits, as measured by the Conner’s Comprehensive Behavior Rating Scale (CBRS) and the attention problems subscale of the Child Behavior Checklist (CBCL), though the magnitude of improvement appeared small. Greater improvements were seen in daytime sleepiness and quality of life. Studies examining cognitive and daytime behavioral function in children using CPAP have demonstrated improvements, even with short CPAP usage (an average of 3 hours per night) [54]. Managing OSA in children with ADHD remains challenging, particularly in terms of early detection and accurate diagnosis. Many symptoms of OSA, such as fatigue, hyperactivity, and inattention, can be mistaken for the core symptoms of ADHD.
Therefore, collaboration between sleep specialists, pediatricians, and psychologists is essential to ensure comprehensive evaluation and timely treatment.
In the future, research on the relationship between OSA and ADHD should focus on further exploring the pathophysiological mechanisms, particularly the effects of intermittent hypoxia and sleep disruption on brain regions associated with ADHD. Additional studies are needed to compare the effectiveness of different OSA treatments, such as surgery and CPAP, on ADHD symptoms, as well as to examine the impact of ADHD medications on sleep. Screening tools for OSA, such as sleep questionnaires, need to be refined for earlier detection in children with ADHD. Moreover, research on genetic and environmental factors affecting both conditions is crucial. The impact of OSA on the cognitive and behavioral development of children with ADHD, as well as its interaction with other psychological disorders, is another potential area of study.
Finally, the application of artificial intelligence in the diagnosis and management of OSA and ADHD could help personalize and enhance treatment effectiveness.
CONCLUSION
The strong relationship between OSA and ADHD in children has been demonstrated through numerous studies. OSA can exacerbate ADHD symptoms through mechanisms of hypoxia and sleep fragmentation, impacting cognitive and behavioral functions. Early diagnosis and appropriate treatment of OSA can significantly improve the quality of life for children with ADHD, with adenotonsillectomy and CPAP being effective treatment options.
However, further research is needed to optimize treatment approaches and better understand the interaction between OSA and ADHD, in order to improve clinical management for this group of children.
CONFLICT OF INTERESTS
None.
REFERENCES
| 1. Anderson DK, Lord C, Risi S, et al. American Psychiatric Association.(2013). Diagnostic and statistical manual of mental disorders . Washington, DC: Author. Linguist Cogn Eff Biling Child Autism Spectr Disord. 2017;21:175. |
| 2. Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5(2):175-186. |
| 3. Willcutt EG. The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics. 2012;9(3):490-499. |
| 4. Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994-1001. doi:10.1542/peds.2014-3482 |
| 5. Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34-46.e2. doi:10.1016/j.jaac.2013.09.001 |
| 6. Sciutto MJ, Eisenberg M. Evaluating the evidence for and against the overdiagnosis of ADHD. J Atten Disord. 2007;11(2):106-113. doi:10.1177/1087054707300094 |
| 7. Coghill D, Soutullo C, d’Aubuisson C, et al. Impact of attention-deficit/hyperactivity disorder on the patient and family: results from a European survey. Child Adolesc Psychiatry Ment Health. 2008;2:1-15. |
| 8. Hodgkins P, Setyawan J, Mitra D, et al. Management of ADHD in children across Europe: patient demographics, physician characteristics and treatment patterns. Eur J Pediatr. 2013;172:895-906. |
| 9. Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep Med. 2010;11(7):652-658. |
| 10. Urbano GL, Tablizo BJ, Moufarrej Y, Tablizo MA, Chen ML, Witmans M. The Link between Pediatric Obstructive Sleep Apnea (OSA) and Attention Deficit Hyperactivity Disorder (ADHD). Children. 2021;8(9):824. doi:10.3390/children8090824 |
| 11. A Clinical Guide to Pediatric Sleep Diagnosis and Management of Sleep Problems (Jodi A. Mindell, Judith A. Owens) (z-lib.org).pdf. |
| 12. Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11(1):1-16. |
| 13. Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Lippincott Williams & Wilkins; 2015. |
| 14. Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in Children With Attention-Deficit/Hyperactivity Disorder: Meta-Analysis of Subjective and Objective Studies. J Am Acad Child Adolesc Psychiatry. 2009;48(9):894-908. |
| 15. Golan N, Shahar E, Ravid S, Pillar G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep. 2004;27(2):261-266. |
| 16. Johnson EO, Roth T. An epidemiologic study of sleep-disordered breathing symptoms among adolescents. Sleep. 2006;29(9):1135-1142. |
| 17. Sciberras E, Heussler H, Berthier J, Lecendreux M. Epidemiology and etiology of medical sleep problems in ADHD. In: Sleep and ADHD. Elsevier; 2019:95-117. |
| 18. Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769-e778. |
| 19. Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. 2007;8(1):18-30. |
| 20. Feng J, Yang, Wang Y, Cao J, Chen B. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: the potential roles played by microglia. Neuropsychiatr Dis Treat. Published online August 2013:1077. doi:10.2147/NDT.S49868 |
| 21. Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. 2007;8(1):18-30. |
| 22. Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24(3):313-320. |
| 23. Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115-1134. |
| 24. Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769-e778. |
| 25. Tran-Minh D, Phi-Thi-Quynh A, Nguyen-Dinh P, Duong-Quy S. Efficacy of obstructive sleep apnea treatment by antileukotriene receptor and surgery therapy in children with adenotonsillar hypertrophy: A descriptive and cohort study. Front Neurol. 2022;13:1008310. doi:10.3389/fneur.2022.1008310 |
| 26. Tran-Minh D, Phi-Quynh A, Nguyen-Dinh P, Duong-Quy S. Study of clinical and polygraphy characteristics of children with tonsillar hypertrophy and OSA. Published online 2021. |
| 27. Tsukada E, Kitamura S, Enomoto M, et al. Prevalence of childhood obstructive sleep apnea syndrome and its role in daytime sleepiness. PLoS ONE. 2018;13(10):e0204409. doi:10.1371/journal.pone.0204409 |
| 28. Ciavarella D, Tepedino M, Chimenti C, et al. Correlation between body mass index and obstructive sleep apnea severity indexes — A retrospective study. Am J Otolaryngol. 2018;39(4):388-391. doi:10.1016/j.amjoto.2018.03.026 |
| 29. Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems.Sleep Med.2000;1:21-32. |
| 30. Borgström A, Nerfeldt P, Friberg D. Questionnaire OSA-18 has poor validity compared to polysomnography in pediatric obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2013;77(11):1864-1868. |
| 31. Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379-388. |
| 32. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473. |
| 33. Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Lippincott Williams & Wilkins; 2015. |
| 34. Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med-JCSM. 2007;3(7):737-747. |
| 35. Duong-Quy, S., Nguyen-Ngoc-Quynh, L., & Nguyen-Huu, H. (2024). 'Personalized medicine': phenotyping pediatric obstructive sleep apnea. Current opinion in pulmonary medicine, 10.1097/MCP.0000000000001119. |
| 36. A Clinical Guide to Pediatric Sleep Diagnosis and Management of Sleep Problems (Jodi A. Mindell, Judith A. Owens) (z-lib.org). |
| 37. Sateia MJ. International classification of sleep disorders. Chest. 2014;146(5):1387-1394. |
| 38. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome | Pediatrics | American Academy of Pediatrics. Accessed October 17, 2024. https://publications.aap.org/pediatrics/article/130/3/576/30284/Diagnosis-and-Management-of-Childhood-Obstructive?autologincheck=redirected |
| 39. Marcus CL, Moore RH, Rosen CL, et al. A Randomized Trial of Adenotonsillectomy for Childhood Sleep Apnea. N Engl J Med. 2013;368(25):2366-2376. |
| 40. Nieminen P, Tolonen U, Löppönen H. Snoring and obstructive sleep apnea in children: a 6-month follow-up study. Arch Otolaryngol Head Neck Surg. 2000;126(4):481-486. doi:10.1001/archotol.126.4.481 |
| 41. Friedman M, Wilson M, Lin HC, Chang HW. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2009;140(6):800-808. doi:10.1016/j.otohns.2009.01.043 |
| 42. Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769-778. doi:10.1542/peds.2005-1837 |
| 43. The effect of tonsillectomy on obstructive sleep apnea: an overview of systematic reviews - PubMed. Accessed August 18, 2023. https://pubmed.ncbi.nlm.nih.gov/29670412/ |
| 44. Song SA, Tolisano AM, Cable BB, Camacho M. Neurocognitive outcomes after pediatric adenotonsillectomy for obstructive sleep apnea: A systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2016;83:205-210. doi:10.1016/j.ijporl.2016.02.011 |
| 45. Aksu H, Günel C, Özgür BG, Toka A, Başak S. Effects of adenoidectomy/adenotonsillectomy on ADHD symptoms and behavioral problems in children. Int J Pediatr Otorhinolaryngol. 2015;79(7):1030-1033. doi:10.1016/j.ijporl.2015.04.018 |
| 46. Amiri S, AbdollahiFakhim S, Lotfi A, Bayazian G, Sohrabpour M, Hemmatjoo T. Effect of adenotonsillectomy on ADHD symptoms of children with adenotonsillar hypertrophy and sleep disordered breathing. Int J Pediatr Otorhinolaryngol. 2015;79(8):1213-1217. doi:10.1016/j.ijporl.2015.05.015 |
| 47. Ahmadi MS, Poorolajal J, Masoomi FS, Haghighi M. Effect of adenotonsillectomy on attention deficit-hyperactivity disorder in children with adenotonsillar hypertrophy: A prospective cohort study. Int J Pediatr Otorhinolaryngol. 2016;86:193-195. doi:10.1016/j.ijporl.2016.05.012 |
| 48. Ayral M, Baylan MY, Kinis V, et al. Evaluation of hyperactivity, attention deficit, and impulsivity before and after adenoidectomy/adenotonsillectomy surgery. J Craniofac Surg. 2013;24(3):731-734. doi:10.1097/SCS.0b013e31828011ea |
| 49. Goldbart AD, Goldman JL, Li RC, Brittian KR, Tauman R, Gozal D. Differential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea syndrome or recurrent infection. Chest. 2004;126(1):13-18. doi:10.1378/chest.126.1.13 |
| 50. Dayyat E, Serpero LD, Kheirandish-Gozal L, et al. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135(5):1142-1149. doi:10.1378/chest.08-2102 |
| 51. Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene Modifier Therapy for Mild Sleep-disordered Breathing in Children. Am J Respir Crit Care Med. 2005;172(3):364-370. doi:10.1164/rccm.200408-1064OC |
| 52. Goldbart AD, Greenberg-Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130(3):e575-580. doi:10.1542/peds.2012-0310 |
| 53. Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714-755. doi:10.1542/peds.2012-1672 |
| 54. Marcus CL, Radcliffe J, Konstantinopoulou S, et al. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185(9):998-1003. doi:10.1164/rccm.201112-2167OC |
FIGURES - TABLE
TABLE 1
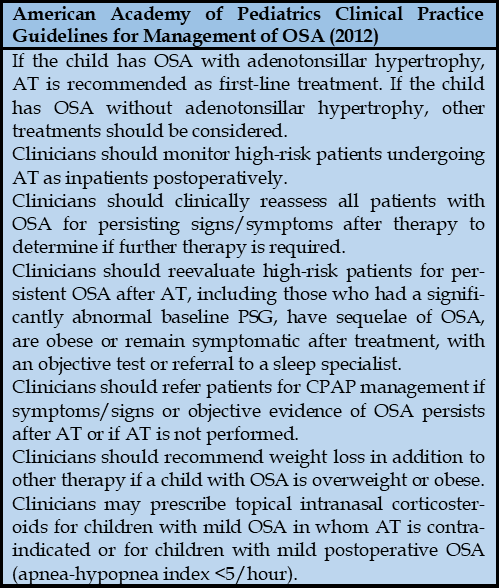 TABLE 2
TABLE 2
REFERENCES
| 1. Anderson DK, Lord C, Risi S, et al. American Psychiatric Association.(2013). Diagnostic and statistical manual of mental disorders . Washington, DC: Author. Linguist Cogn Eff Biling Child Autism Spectr Disord. 2017;21:175. |
| 2. Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5(2):175-186. |
| 3. Willcutt EG. The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics. 2012;9(3):490-499. |
| 4. Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994-1001. doi:10.1542/peds.2014-3482 |
| 5. Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34-46.e2. doi:10.1016/j.jaac.2013.09.001 |
| 6. Sciutto MJ, Eisenberg M. Evaluating the evidence for and against the overdiagnosis of ADHD. J Atten Disord. 2007;11(2):106-113. doi:10.1177/1087054707300094 |
| 7. Coghill D, Soutullo C, d’Aubuisson C, et al. Impact of attention-deficit/hyperactivity disorder on the patient and family: results from a European survey. Child Adolesc Psychiatry Ment Health. 2008;2:1-15. |
| 8. Hodgkins P, Setyawan J, Mitra D, et al. Management of ADHD in children across Europe: patient demographics, physician characteristics and treatment patterns. Eur J Pediatr. 2013;172:895-906. |
| 9. Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep Med. 2010;11(7):652-658. |
| 10. Urbano GL, Tablizo BJ, Moufarrej Y, Tablizo MA, Chen ML, Witmans M. The Link between Pediatric Obstructive Sleep Apnea (OSA) and Attention Deficit Hyperactivity Disorder (ADHD). Children. 2021;8(9):824. doi:10.3390/children8090824 |
| 11. A Clinical Guide to Pediatric Sleep Diagnosis and Management of Sleep Problems (Jodi A. Mindell, Judith A. Owens) (z-lib.org).pdf. |
| 12. Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11(1):1-16. |
| 13. Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Lippincott Williams & Wilkins; 2015. |
| 14. Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in Children With Attention-Deficit/Hyperactivity Disorder: Meta-Analysis of Subjective and Objective Studies. J Am Acad Child Adolesc Psychiatry. 2009;48(9):894-908. |
| 15. Golan N, Shahar E, Ravid S, Pillar G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep. 2004;27(2):261-266. |
| 16. Johnson EO, Roth T. An epidemiologic study of sleep-disordered breathing symptoms among adolescents. Sleep. 2006;29(9):1135-1142. |
| 17. Sciberras E, Heussler H, Berthier J, Lecendreux M. Epidemiology and etiology of medical sleep problems in ADHD. In: Sleep and ADHD. Elsevier; 2019:95-117. |
| 18. Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769-e778. |
| 19. Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. 2007;8(1):18-30. |
| 20. Feng J, Yang, Wang Y, Cao J, Chen B. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: the potential roles played by microglia. Neuropsychiatr Dis Treat. Published online August 2013:1077. doi:10.2147/NDT.S49868 |
| 21. Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. 2007;8(1):18-30. |
| 22. Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24(3):313-320. |
| 23. Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115-1134. |
| 24. Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769-e778. |
| 25. Tran-Minh D, Phi-Thi-Quynh A, Nguyen-Dinh P, Duong-Quy S. Efficacy of obstructive sleep apnea treatment by antileukotriene receptor and surgery therapy in children with adenotonsillar hypertrophy: A descriptive and cohort study. Front Neurol. 2022;13:1008310. doi:10.3389/fneur.2022.1008310 |
| 26. Tran-Minh D, Phi-Quynh A, Nguyen-Dinh P, Duong-Quy S. Study of clinical and polygraphy characteristics of children with tonsillar hypertrophy and OSA. Published online 2021. |
| 27. Tsukada E, Kitamura S, Enomoto M, et al. Prevalence of childhood obstructive sleep apnea syndrome and its role in daytime sleepiness. PLoS ONE. 2018;13(10):e0204409. doi:10.1371/journal.pone.0204409 |
| 28. Ciavarella D, Tepedino M, Chimenti C, et al. Correlation between body mass index and obstructive sleep apnea severity indexes — A retrospective study. Am J Otolaryngol. 2018;39(4):388-391. doi:10.1016/j.amjoto.2018.03.026 |
| 29. Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems.Sleep Med.2000;1:21-32. |
| 30. Borgström A, Nerfeldt P, Friberg D. Questionnaire OSA-18 has poor validity compared to polysomnography in pediatric obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2013;77(11):1864-1868. |
| 31. Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379-388. |
| 32. Aurora RN, Lamm CI, Zak RS, et al. Practice parameters for the non-respiratory indications for polysomnography and multiple sleep latency testing for children. Sleep. 2012;35(11):1467-1473. |
| 33. Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Lippincott Williams & Wilkins; 2015. |
| 34. Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med-JCSM. 2007;3(7):737-747. |
| 35. Duong-Quy, S., Nguyen-Ngoc-Quynh, L., & Nguyen-Huu, H. (2024). 'Personalized medicine': phenotyping pediatric obstructive sleep apnea. Current opinion in pulmonary medicine, 10.1097/MCP.0000000000001119. |
| 36. A Clinical Guide to Pediatric Sleep Diagnosis and Management of Sleep Problems (Jodi A. Mindell, Judith A. Owens) (z-lib.org). |
| 37. Sateia MJ. International classification of sleep disorders. Chest. 2014;146(5):1387-1394. |
| 38. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome | Pediatrics | American Academy of Pediatrics. Accessed October 17, 2024. https://publications.aap.org/pediatrics/article/130/3/576/30284/Diagnosis-and-Management-of-Childhood-Obstructive?autologincheck=redirected |
| 39. Marcus CL, Moore RH, Rosen CL, et al. A Randomized Trial of Adenotonsillectomy for Childhood Sleep Apnea. N Engl J Med. 2013;368(25):2366-2376. |
| 40. Nieminen P, Tolonen U, Löppönen H. Snoring and obstructive sleep apnea in children: a 6-month follow-up study. Arch Otolaryngol Head Neck Surg. 2000;126(4):481-486. doi:10.1001/archotol.126.4.481 |
| 41. Friedman M, Wilson M, Lin HC, Chang HW. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2009;140(6):800-808. doi:10.1016/j.otohns.2009.01.043 |
| 42. Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769-778. doi:10.1542/peds.2005-1837 |
| 43. The effect of tonsillectomy on obstructive sleep apnea: an overview of systematic reviews - PubMed. Accessed August 18, 2023. https://pubmed.ncbi.nlm.nih.gov/29670412/ |
| 44. Song SA, Tolisano AM, Cable BB, Camacho M. Neurocognitive outcomes after pediatric adenotonsillectomy for obstructive sleep apnea: A systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2016;83:205-210. doi:10.1016/j.ijporl.2016.02.011 |
| 45. Aksu H, Günel C, Özgür BG, Toka A, Başak S. Effects of adenoidectomy/adenotonsillectomy on ADHD symptoms and behavioral problems in children. Int J Pediatr Otorhinolaryngol. 2015;79(7):1030-1033. doi:10.1016/j.ijporl.2015.04.018 |
| 46. Amiri S, AbdollahiFakhim S, Lotfi A, Bayazian G, Sohrabpour M, Hemmatjoo T. Effect of adenotonsillectomy on ADHD symptoms of children with adenotonsillar hypertrophy and sleep disordered breathing. Int J Pediatr Otorhinolaryngol. 2015;79(8):1213-1217. doi:10.1016/j.ijporl.2015.05.015 |
| 47. Ahmadi MS, Poorolajal J, Masoomi FS, Haghighi M. Effect of adenotonsillectomy on attention deficit-hyperactivity disorder in children with adenotonsillar hypertrophy: A prospective cohort study. Int J Pediatr Otorhinolaryngol. 2016;86:193-195. doi:10.1016/j.ijporl.2016.05.012 |
| 48. Ayral M, Baylan MY, Kinis V, et al. Evaluation of hyperactivity, attention deficit, and impulsivity before and after adenoidectomy/adenotonsillectomy surgery. J Craniofac Surg. 2013;24(3):731-734. doi:10.1097/SCS.0b013e31828011ea |
| 49. Goldbart AD, Goldman JL, Li RC, Brittian KR, Tauman R, Gozal D. Differential expression of cysteinyl leukotriene receptors 1 and 2 in tonsils of children with obstructive sleep apnea syndrome or recurrent infection. Chest. 2004;126(1):13-18. doi:10.1378/chest.126.1.13 |
| 50. Dayyat E, Serpero LD, Kheirandish-Gozal L, et al. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135(5):1142-1149. doi:10.1378/chest.08-2102 |
| 51. Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene Modifier Therapy for Mild Sleep-disordered Breathing in Children. Am J Respir Crit Care Med. 2005;172(3):364-370. doi:10.1164/rccm.200408-1064OC |
| 52. Goldbart AD, Greenberg-Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130(3):e575-580. doi:10.1542/peds.2012-0310 |
| 53. Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714-755. doi:10.1542/peds.2012-1672 |
| 54. Marcus CL, Radcliffe J, Konstantinopoulou S, et al. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185(9):998-1003. doi:10.1164/rccm.201112-2167OC |
ARTICLE INFO DOI: 10.12699/jfvpulm.15.47.2024.1
Conflict of Interest
Non
Date of manuscript receiving
25/06/2024
Date of publication after correction
25/11/2024
Article citation
Mai Nguyen-Thi-Phuong, Mai Nguyen-Thi-Thanh, Sy Duong-Quy. Obstructive sleep apnea in children with attention deficit hyperactivity disorder: A literature review. J Func Vent Pulm 2024;47(15):1-9